Preparation method of polythioether corrosion inhibitor containing cobaltocene cation side group and used corrosion inhibitor system
A technology of cobaltene cation and polysulfide, which can be used in coatings, anti-corrosion coatings, etc., and can solve the problems of poor film-forming ability and few attachment sites of small-molecule corrosion inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
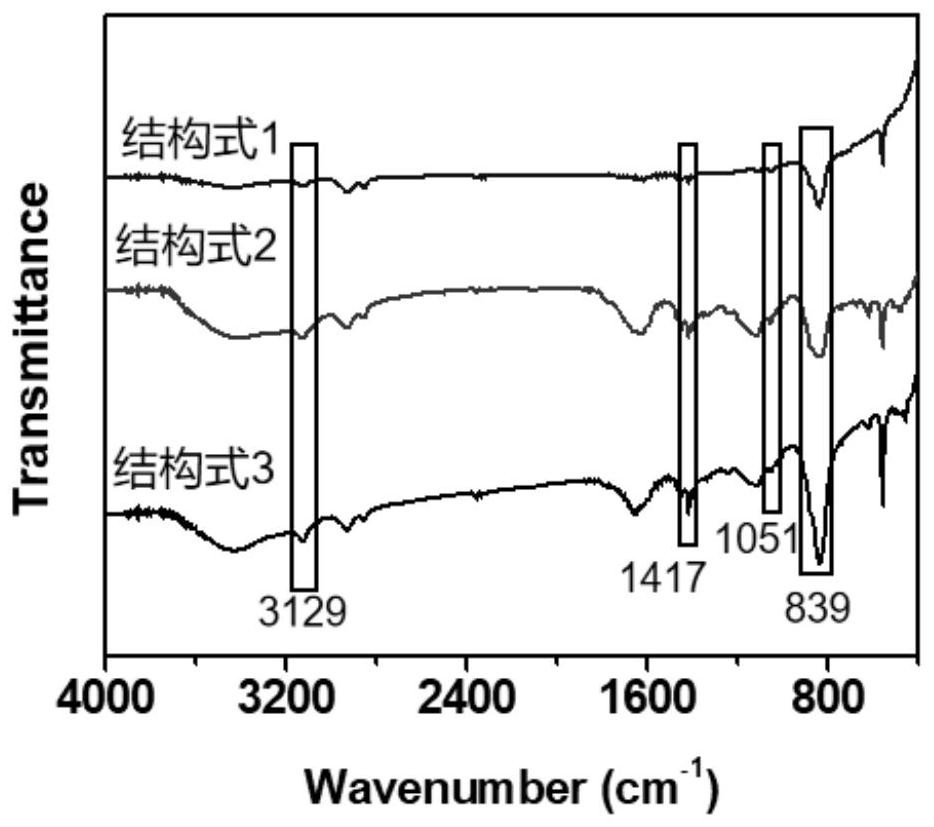
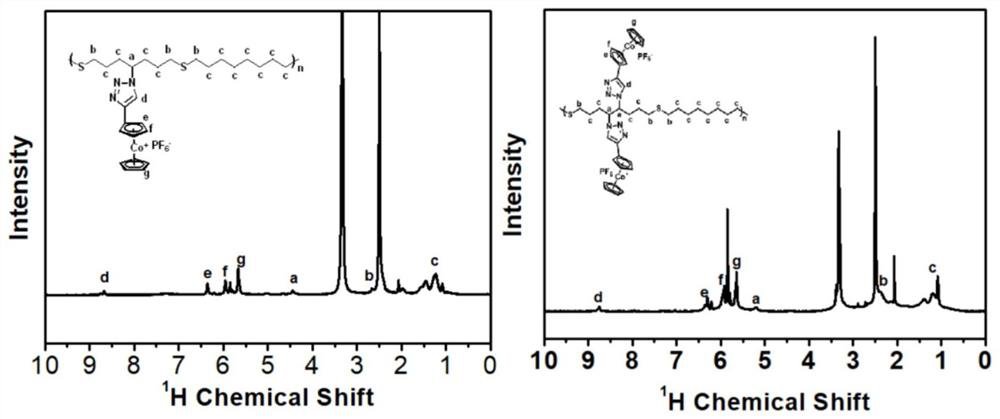
[0043] Example 1: When X - = Cl - When the preparation of structural formula 1
[0044] Dissolve 1,6-heptadien-4-ol in an appropriate amount of dichloromethane, add 1-3 parts of triethylamine, 2-6 parts of methanesulfonic anhydride, 0.5-1 parts 4-Dimethylaminopyridine, low temperature reaction 1-4h, room temperature reaction 24-48h. Add water to quench the reaction, add an equal amount of dichloromethane for extraction and separation, remove the organic phase, and obtain 1,6-heptadiene-4-methylsulfonate through column separation and purification.
[0045] Put 1 part of 1,6-heptadiene-4-methylsulfonate and 1 part of octanedithiol in a vial, add 0.01-0.05 part of photoinitiator, and irradiate with 365nm ultraviolet light for 0.5-2h. The product was dissolved in tetrahydrofuran and precipitated twice in methanol to obtain a polythioether containing methylsulfonate side groups.
[0046] Dissolve the polythioether containing methylsulfonate side groups in a small amount of N,N-...
Embodiment 2
[0049] Example 2: When X - = Cl - When the preparation of structural formula 2
[0050] Dissolve 1-3 parts of allyl bromide, 1-5 parts of potassium iodide and 1-3 parts of stannous chloride hydrate in 10-30mL water and place in a round bottom flask, and dissolve 1-3 parts of glyoxal in 5 -10mL water and dropwise into the above round bottom flask, react at room temperature for 24-48h, add 5-20mL 1M HCl solution, add ethyl acetate for extraction and separation three times, add appropriate amount of sodium thiosulfate hydrate for decolorization, and then use Ethyl acetate was extracted and separated once, and the organic phase was removed and dried to obtain 1,7-octadiene-4,5-diol.
[0051] Dissolve 1,7-octadiene-4,5-diol in an appropriate amount of dichloromethane, add 1-3 parts of triethylamine, 2-6 parts of methanesulfonic anhydride, 0.5 - 1 part of 4-dimethylaminopyridine, react at low temperature for 1-4h, and at room temperature for 24-48h. Add water to quench the react...
Embodiment 3
[0056] Example 3: When X - = Cl - When the preparation of structural formula 3
[0057] Dissolve 1 part of diethyl diallylmalonate in 5-15 mL of ether and place in a round bottom flask, disperse 1-3 parts of lithium aluminum hydride in 10-20 mL of ether and slowly add the above In a round bottom flask, stir at room temperature for 12-24h. Add an appropriate amount of water to quench the reaction, filter to remove the precipitate, add an appropriate amount of dichloromethane for multiple extractions, remove the organic phase, and dry to obtain diallyl propylene glycol.
[0058] Dissolve diallyl propylene glycol in an appropriate amount of dichloromethane, add 1-3 parts of triethylamine, 2-6 parts of methanesulfonic anhydride, and 0.5-1 part of 4-dimethylaminopyridine in a nitrogen atmosphere and ice bath , low temperature reaction 1-4h, room temperature reaction 24-48h. Water was added to quench the reaction, an equal amount of dichloromethane was added to extract and separ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com