Preparation method of 2-aminopropanol
An aminopropanol, liquid ammonia technology, applied in the preparation of aminohydroxy compounds, preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of large amount of waste water, high equipment requirements, low product selectivity, etc., and achieve the catalyst life. Improve, high atom utilization, reduce the effect of ring-opening polymerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
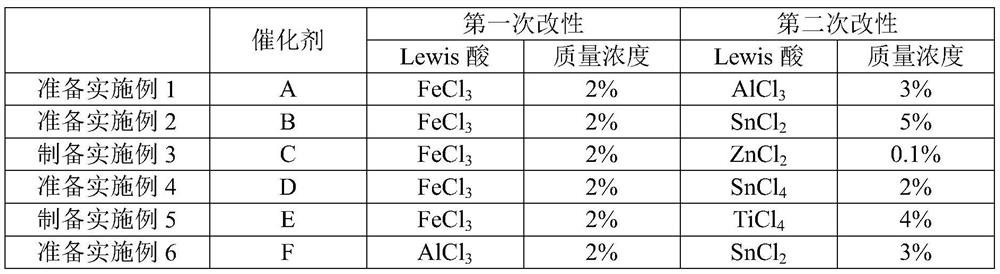
[0050] [Preparation Example 1] Preparation of FeCl 3 , AlCl 3 Modified Macroporous Styrenic Strongly Acidic Cation Exchange Resin
[0051] a. Resin pretreatment: wash the macroporous styrene-based strongly acidic cation exchange resin with absolute ethanol for 12 hours, and then wash with deionized water until the effluent is colorless; continuously purge and dry with nitrogen at 70°C until constant weight.
[0052] b. The first modification of Lewis acid:
[0053] The preparation mass concentration is 2% FeCl 3 Ethanol solution, make the pretreated macroporous styrene-based strongly acidic cation exchange resin fully react with it for 12h, the reaction temperature is 50°C, and carry out the first modification; then wash 5 times with deionized water, and then wash 5 times with acetone times, and finally washed with deionized water until chlorine-free (using AgNO with a mass fraction of 1%) 3 The solution was titrated with the cleaning solution, and no precipitation after t...
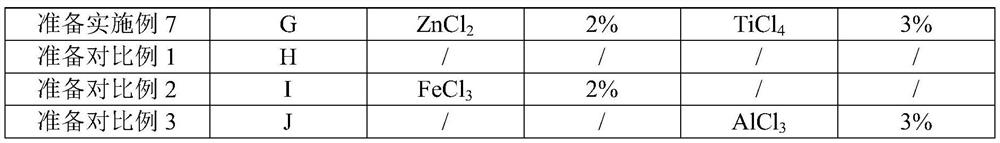
Embodiment 2-7 and comparative example 1-3
[0067] The other examples and comparative examples were carried out according to the steps substantially the same as in Example 1, the only difference being that the reaction conditions and parameters shown in Table 2 were different.
[0068] Catalyst service life evaluation:
[0069] Catalysts A, F, G, and H were respectively subjected to a continuous catalytic efficiency test according to the operation method in Example 1, and the conversion rate of the raw material and the selectivity of the product were recorded after the catalyst was running for a long time, as shown in Table 4.
[0070] Different reaction conditions and parameters in table 2, each embodiment and comparative examples
[0071]
[0072]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com