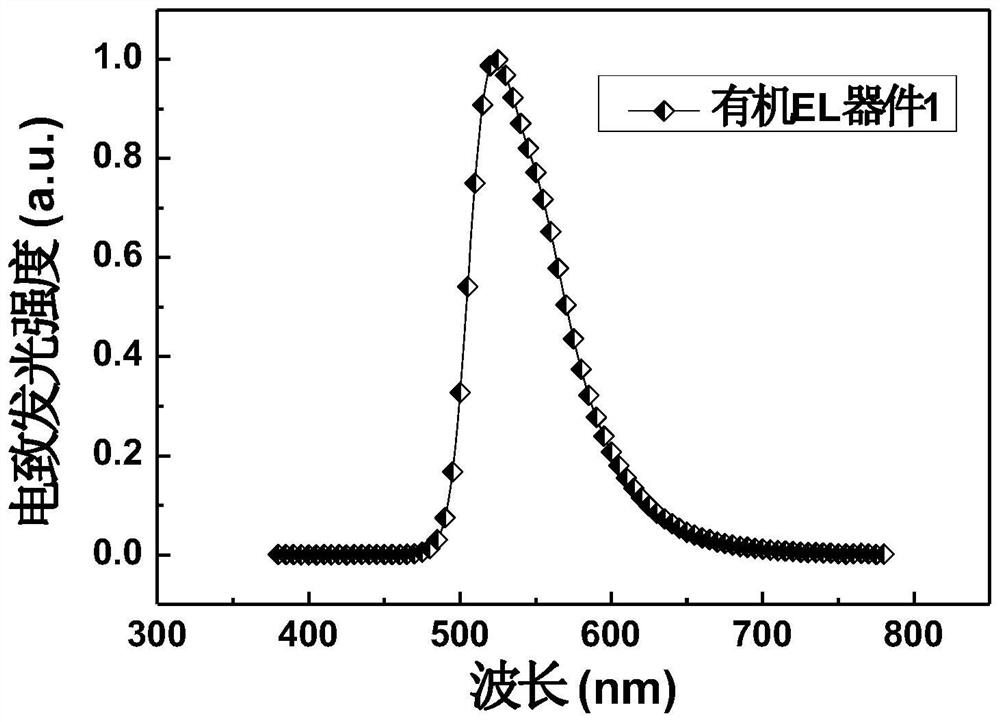
Organic electroluminescent material based on tetraphenylhydrazine derivative and electronic device thereof
A technology of tetraphenylhydrazine and its derivatives, applied in the field of organic electroluminescent materials and electronic devices, can solve the problems of short life, low efficiency of electroluminescent materials, high cost, etc., and achieve improved life, low cost and long life Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Embodiment 1: the synthesis of compound 18
[0095] (Synthesis of Intermediate 1-1)
[0096] The synthetic route of intermediate 1-1 is as follows:
[0097]
[0098] Under nitrogen protection, potassium iodide (KI, 332 mg, 2 mmol), potassium periodate (KIO 4 , 6.9 g, 30 mmol), 4-bromodiphenylamine (5.0 g, 20 mmol) and 50 mL of anhydrous acetonitrile (MeCN), and react overnight at room temperature. After the reaction was completed, the solvent was evaporated under reduced pressure, and the crude product was further purified by column chromatography (petroleum ether:dichloromethane=4:1 (V / V)). After distilling off the solvent, 4.2 g of a white solid was obtained, with a yield of 85%. MS (EI): m / z: 494.10 [M+]. Anal.calcd for C24H18Br2N2(%): C 58.33, H 3.67, N 5.67; found: C 58.30, H3.70, N5.65.
[0099] (Synthesis of compound 18)
[0100] The synthetic route of compound 18 is as follows:
[0101]
[0102] Under nitrogen protection, intermediate 1-1 (2.5g, 5mm...
Embodiment 2
[0103] Embodiment 2: the synthesis of compound 12
[0104] (Synthesis of compound 12)
[0105] The synthetic route of compound 12 is as follows:
[0106]
[0107] Under nitrogen protection, intermediate 1-1 (2.5g, 5mmol), 7,7-dimethyl-5,7-dihydroindeno[2,1-b]carbazole were added successively in a 250mL Schlenk bottle ( 3.1 g, 11 mmol), palladium acetate (11 mg, 0.05 mmol), tri-tert-butylphosphine tetrafluoroborate (29 mg, 0.1 mmol), sodium tert-butoxide (1.9 g, 20 mmol), and 120 mL of toluene, stirred at reflux Reaction 12 Hour. After the reaction was complete, the solvent was evaporated, and the residue was dissolved with 200 mL of dichloromethane and 50 mL of water, washed with water, and the organic layer was separated. The aqueous layer was extracted twice with 15 mL of dichloromethane, and the organic layers were combined. After distilling off the solvent, the residue was separated by column chromatography (petroleum ether:dichloromethane=2:1 (V / V)). The solvent wa...
Embodiment 3
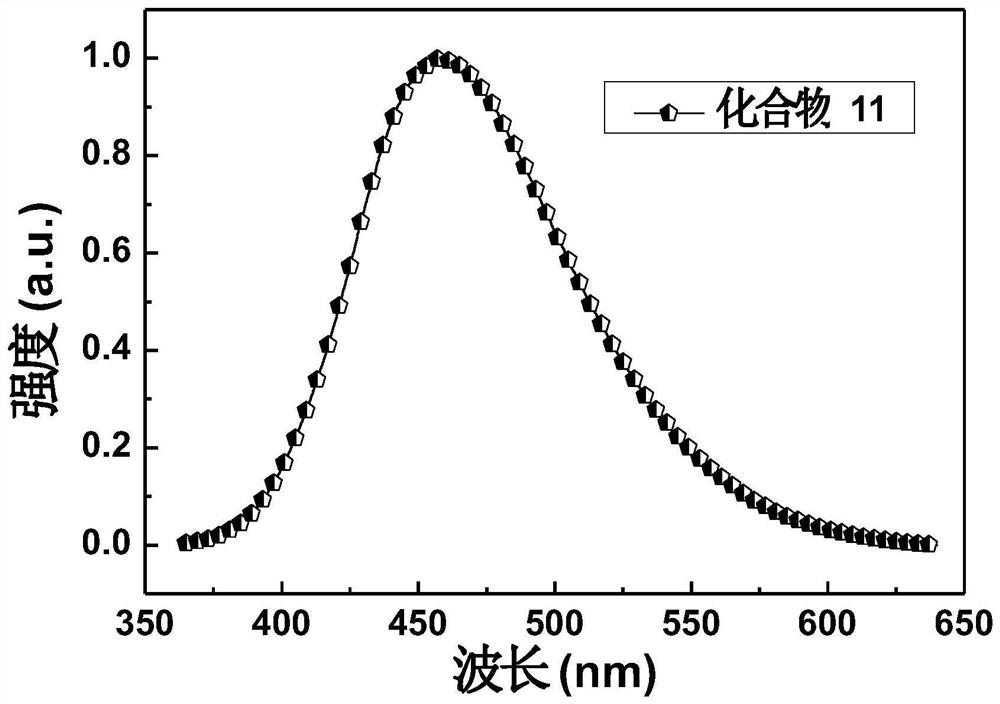
[0108] Embodiment 3: the synthesis of compound 11
[0109] (Synthesis of Intermediate 1-2)
[0110] The synthetic route of intermediate 1-2 is shown below:
[0111]
[0112] Under nitrogen protection, potassium iodide (KI, 332 mg, 2 mmol), potassium periodate (KIO 4 , 6.9g, 30mmol), 4-bromodiphenylamine (2.5g, 10mmol), diphenylamine (1.7g, 10mmol) and 50mL of anhydrous acetonitrile (MeCN), and react overnight at room temperature. After the reaction was completed, the solvent was evaporated under reduced pressure, and the crude product was further purified by column chromatography (petroleum ether:dichloromethane=4:1 (V / V)). After distilling off the solvent, 2.9 g of a white solid was obtained, with a yield of 70%. MS (EI): m / z: 414.78 [M+]. Anal.calcd for C24H19BrN2(%): C 69.41, H 4.61, N 6.74; found: C 69.38, H 4.65, N 6.75.
[0113] (Synthesis of compound 11)
[0114] The synthetic route of compound 11 is as follows:
[0115]
[0116] Under nitrogen, add interme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com