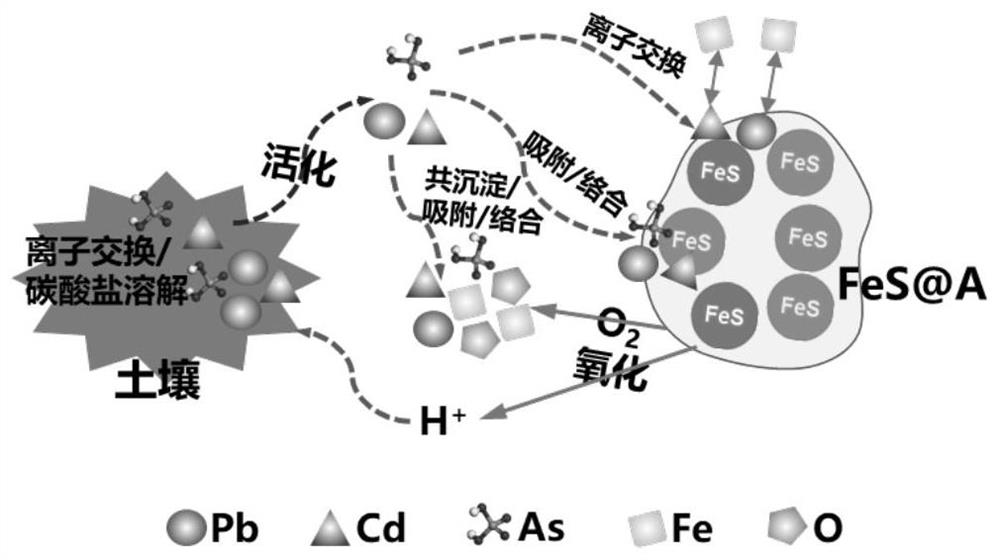
Heavy metal stabilizing material in polluted soil as well as preparation method and application of heavy metal stabilizing material
A technology for polluting soil and heavy metals, applied in the field of soil treatment, can solve the problems of inability to effectively reduce the oxidation rate of ferrous sulfide, unstable organic ligands, low heavy metal content, etc., to reduce mobility and environmental risks, reduce material costs, The effect of high mass transfer rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: Preparation of Heavy Metal Stabilized Materials
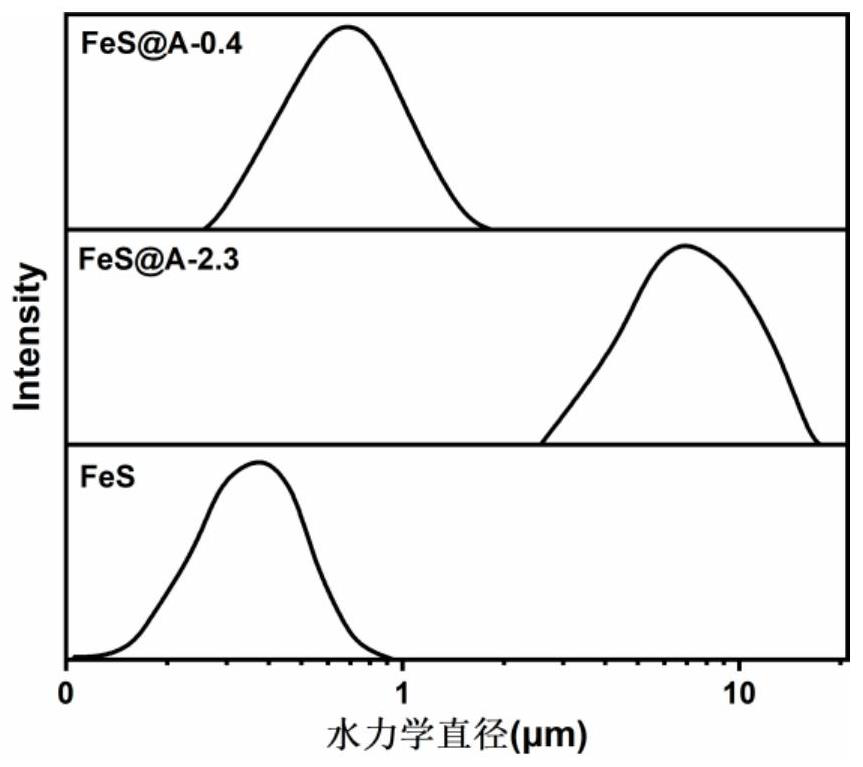
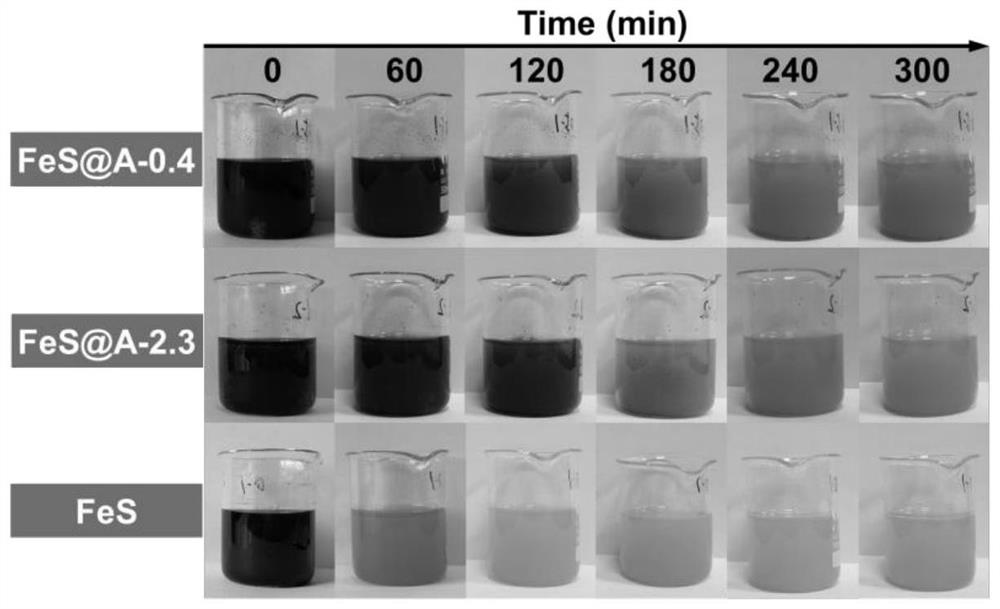
[0059] (1) Heavy metal stabilized material with a mass ratio of calcium alginate to ferrous sulfide of 0.4:1 (FeS@A-0.4)
[0060] Dissolve 1.44g of sodium sulfide nonahydrate and 0.2g of sodium alginate in 40mL of anaerobic water in sequence (after sodium sulfide is dissolved and hydrolyzed, the pH of the solution rises, which is conducive to the dissolution of sodium alginate, so it is necessary to dissolve sodium sulfide first, and then dissolve the seaweed sodium alginate), under the protection of nitrogen, the stirring speed was 300rpm to obtain a sodium alginate-sodium sulfide mixed solution.
[0061] Dissolve 1.67g of ferrous sulfate heptahydrate in 60mL of anaerobic water, and under the protection of an inert gas, stir continuously at a rotation speed of 300rpm to obtain a ferrous sulfate solution.
[0062] After sucking the sodium alginate-sodium sulfide mixed solution into the syringe, add 40 mL of t...
Embodiment 2
[0078] Example 2: Preparation of Heavy Metal Stabilized Materials
[0079] (1) Heavy metal stabilized material (FeS@A-2.3-H) with thick coating layer calcium alginate and ferrous sulfide mass ratio of 2.3:1
[0080] 0.768g of sodium sulfide nonahydrate and 0.6g of sodium alginate were successively dissolved in 10mL of anaerobic water, and stirred continuously at a speed of 300rpm under nitrogen protection to obtain a sodium alginate-sodium sulfide mixed solution.
[0081] Dissolve 0.890 g of ferrous sulfate heptahydrate in 90 mL of anaerobic water, and under nitrogen protection, stir continuously at a rotation speed of 200 rpm to obtain a ferrous sulfate solution.
[0082] After sucking the sodium alginate-sodium sulfide mixed solution into the syringe, add 10 mL of the sodium alginate-ferrous sulfide mixed solution dropwise at a speed of 6 mL / min to 90 mL of the continuously stirring ferrous sulfide solution with a 1 mm needle under nitrogen protection. The height from the l...
Embodiment 3
[0095] Example 3: Application of heavy metal stabilization materials to stabilize heavy metals in soil
[0096] Take 20g of lead, cadmium and arsenic compound polluted soil in a 50mL glass beaker. The soil pH is 7.84. The content is 16.1mmol / kg, and the required amount of ferrous sulfide is 18.2mmol / kg.
[0097] To the beaker containing the above soil, add: 6.1 mL of the FeS@A-0.4 suspension prepared in Example 1 (the concentration of ferrous sulfide is 60 mM, the thickness of the calcium alginate covering layer is 0.05-10.5 μm), FeS @A-2.3 suspension 11.4mL (concentration of ferrous sulfide is 32mM, thickness of calcium alginate covering layer is 0.3-8.7μm), FeS@A-1.0-L suspension 8.0mL prepared in Example 2 (sulfurized The concentration of ferrous iron is 46mM, and the thickness of the calcium alginate covering layer is 0.9-10.5μm). For each sample, stir with a blade stirrer at a speed of 50rmp for 30min. Cover the soil, create an anoxic environment, and continue to mainta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydrated particle size | aaaaa | aaaaa |
| Hydrated particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com