Oral care composition as well as preparation method and application thereof
An oral care and composition technology, applied in the field of biomaterials and oral care, can solve the problems of unsustainable drug release, short residence time, and low specificity, so as to reduce toxic and side effects, reduce the occurrence of bacterial drug resistance, The effect of preventing tooth hard tissue defect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
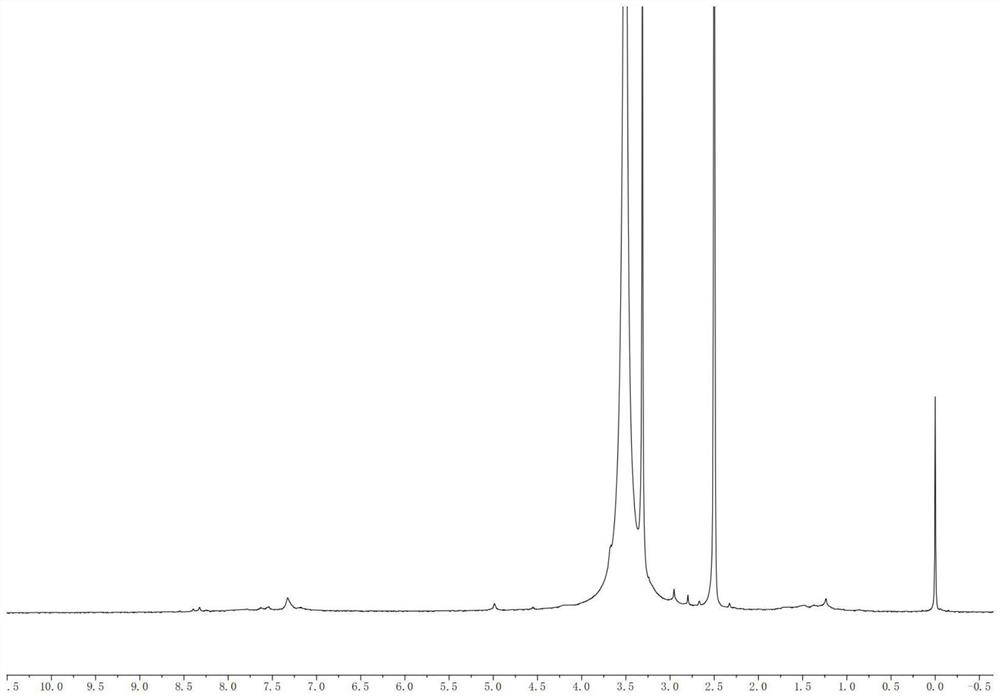
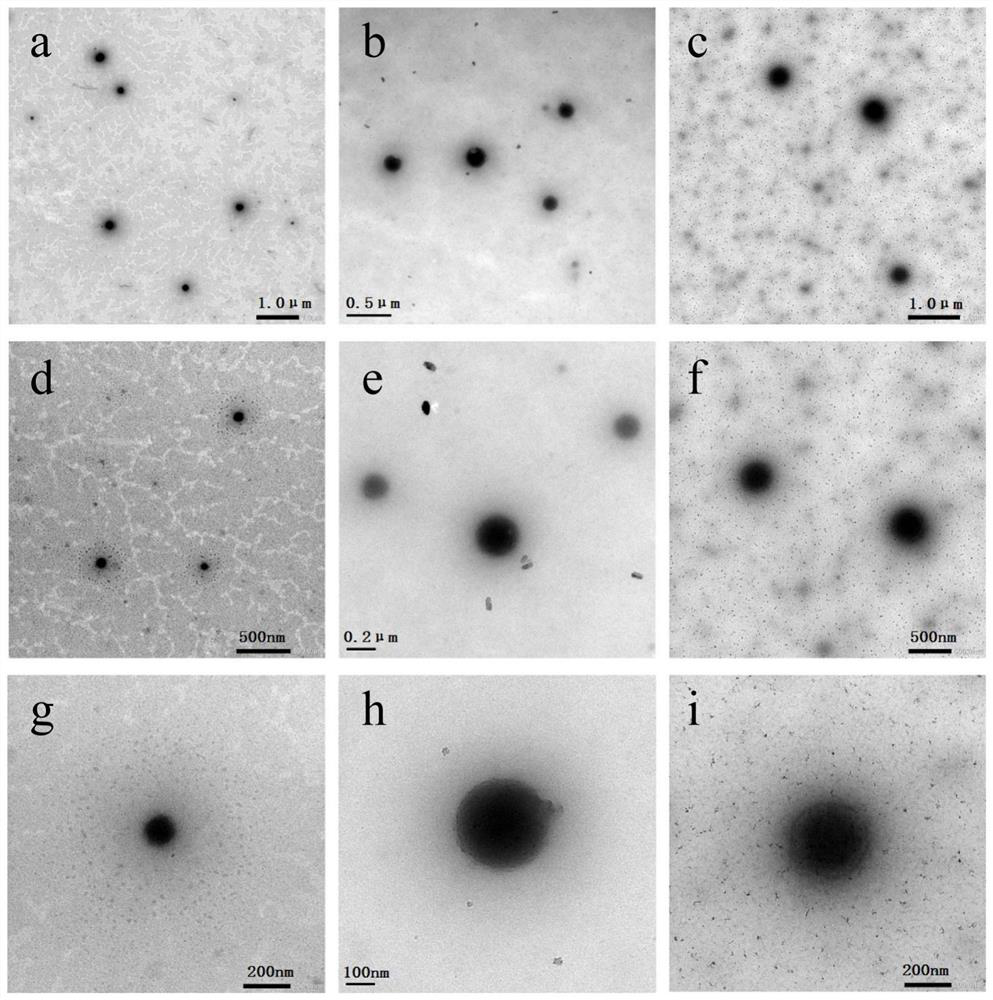
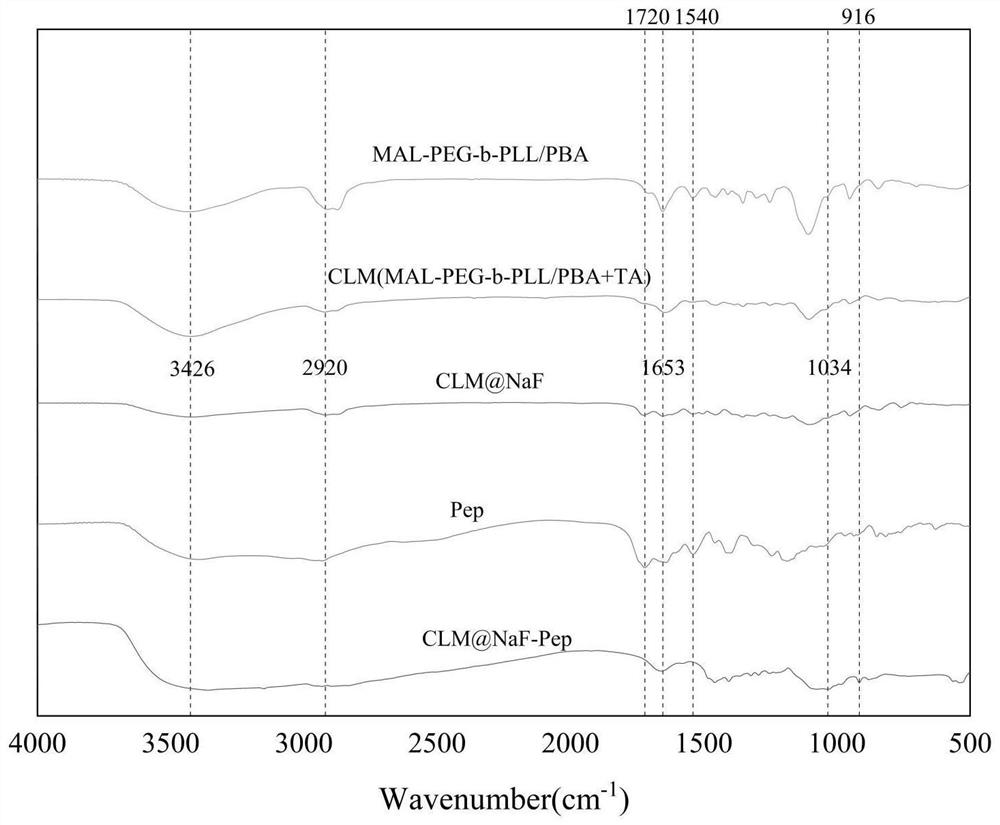
[0037] Example 1: Preparation of micellar composites loaded with tannic acid and sodium fluoride and linked to sialoprotein polypeptides
[0038] (1) Preparation of water-soluble polymer:
[0039] Polyethylene glycol diamine (CAS No.: 24991-53-5, NH 2 C 2 H 2 -(CH 2 O) n -C 2 H 2 NH 2 ) co-reacted with 3-maleimidopropionic acid (MAL-), and MAL was modified on the polymer to obtain the product MAL-PEG-NH 2 .
[0040] ε-(benzyloxycarbonyl)-L-lysine N-carboxyanhydride (ε-benzyloxycarbonyl-L-lysine-N-carboxy-cyclic anhydride, Lys(Z)-NCA) (1.96 g, 6.4 mmol) was dissolved in 30mL of N,N-dimethylformamide (DMF), by adding MAL-PEG-NH 2 (2.0 g, 0.4 mmol), and stirred for 72 h under dry argon at 35 °C to allow polymerization to occur. The solvent was then rotary evaporated and the product was dissolved in 25 mL of CHCl 3 , placed in excess ether for precipitation to obtain the product MAL-PEG-b-PZLL.
[0041] In order to remove the benzyl protecting group, remove the protecti...
Embodiment 2
[0052] Example 2: Drug pH Controlled Release Test
[0053] The drug release from CLM@NaF-Pep under different pH conditions was detected by high performance liquid chromatography and ion exchange chromatography. 2 mL of the freshly prepared CLM@NaF-Pep dispersion was transferred into a dialysis bag (MWCO 3500), then soaked in 15 mL of 10 mM PBS with different pH, and shaken at 37 °C (rotation speed 100 r / min). At fixed time intervals (every 0.5h, 1h, 2h, 4h, 8h, 12h, 24h), 1 mL of dialysate was collected until 24h, and an equal volume of fresh buffer was added every time the dialysate was collected to keep the total volume of dialysate. constant. The contents of tannic acid and sodium fluoride in the dialysate were detected by high performance liquid chromatography and ion exchange chromatography, respectively. Three parallel control groups were set up in all experiments, the average value was calculated and compared, and the experiment was repeated three times.
[0054] The...
Embodiment 3
[0055] Example 3: Test for the effect of inhibiting the growth of Streptococcus mutans
[0056] The Streptococcus mutans cultured to the exponential phase was collected by centrifugation for 3 min (5000 rpm), and diluted to 10 using BHI medium. 6 CFU / mL to obtain bacterial suspension.
[0057] CLM@NaF-Pep was added to BHI medium with pH of 7.4, 6.5 and 5.0, respectively, to prepare the experimental group dispersion; PBS was used as the blank control solution, and chlorhexidine (CHX) was used as the positive control solution. Aliquot into 96-well plates (160 μL per well). Then, 40 μL of the above bacterial suspension was added to the dispersion liquid of the experimental group and the solution of the control group and mixed well. The 96-well plate was placed in a 37°C incubator, and incubated for 0.5, 1, 2, 4, 8, 12, and 24 hours, respectively, and then 100 μL of culture medium was taken from each group and inoculated into a new 96-well plate. The antibacterial activity was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com