Preparation method of high-compaction lithium iron phosphate
A real lithium iron phosphate, high-voltage technology, applied in chemical instruments and methods, phosphorus compounds, nanotechnology for materials and surface science, etc., can solve the problem that lithium iron phosphate cannot improve both the electronic conductivity and ion conductivity of lithium iron phosphate Improve the intrinsic conductivity, improve the gram capacity, and improve the compacted density and conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) According to a total of 1.5 g of LiFe 0.95 Ca 0.05 PO 4 The stoichiometric ratios of lithium acetate, phosphoric acid, ferric chloride and calcium chloride are weighed respectively;
[0034] (2) Lithium acetate is added to the absolute ethanol containing 5% (wt) polyethylene glycol, and is continuously stirred until a clear clear liquid is formed;
[0035] (3) Add ammonium dihydrogen phosphate, iron oxalate and calcium chloride to the above clear liquid, stir well for 5 hours to obtain a sol, transfer the sol to a watch glass, dry at 80°C for 15 hours to obtain a gel, and grind the gel into powder;
[0036] (4) calcining the powder in a reducing atmosphere, calcining at 500 ℃ for 2 hours, and then calcining at 800 ℃ for 8 hours;
[0037] (5) Naturally cooled to room temperature, and ground to obtain powdery lithium iron phosphate.
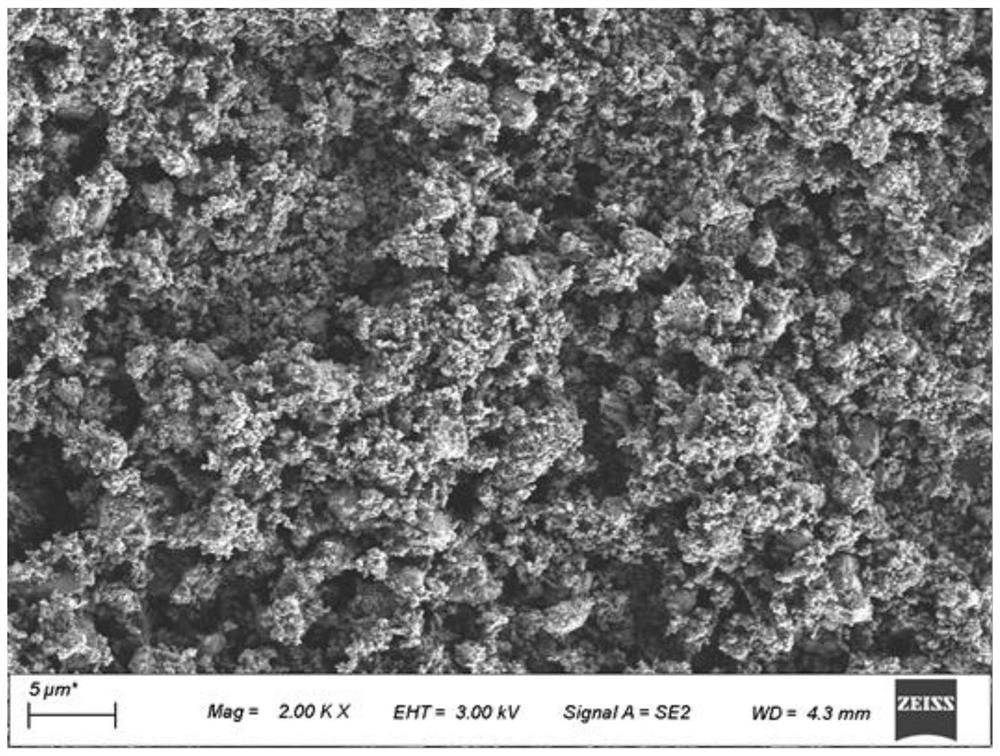
[0038] The morphology of the calcined lithium iron phosphate powder in Example 1 was observed by SEM, as shown in figure 1 , fi...
Embodiment 2
[0040] (1) According to a total of 1.5 g of LiFe 0.97 Zn 0.03 PO 4 The stoichiometric ratios of lithium hydroxide, phosphoric acid, ferric chloride and zinc nitrate are weighed respectively;
[0041] (2) Lithium hydroxide is added to the absolute ethanol containing 2% (wt) cetyl trimethyl ammonium bromide, stirring constantly, until transparent clear liquid is formed;
[0042] (3) adding phosphoric acid, ferric chloride and zinc nitrate to the above clear liquid, fully stirring for 4h to obtain a sol, transferring the sol to a watch glass, drying at 85°C for 13h to obtain a gel, and grinding into powder;
[0043](4) calcining the powder in a reducing atmosphere, calcining at 400°C for 3h, and then calcining at 600°C for 10h;
[0044] (5) Naturally cooled to room temperature, and ground to obtain powdery lithium iron phosphate.
Embodiment 3
[0046] (1) According to a total of 1.5 g of LiFe 0.98 Zn 0.03 PO 4 The stoichiometric ratios of lithium hydroxide, phosphoric acid, ferric chloride and zinc nitrate are weighed respectively;
[0047] (2) Lithium hydroxide is added to the absolute ethanol containing 2% (wt) cetyl trimethyl ammonium bromide, stirring constantly, until transparent clear liquid is formed;
[0048] (3) adding phosphoric acid, ferric chloride and zinc nitrate to the above clear liquid, fully stirring for 4h to obtain a sol, transferring the sol to a watch glass, drying at 85°C for 13h to obtain a gel, and grinding into powder;
[0049] (4) calcining the powder in a reducing atmosphere, calcining at 350°C for 3h, and then calcining at 750°C for 10h;
[0050] (5) Naturally cooled to room temperature, and ground to obtain powdery lithium iron phosphate.
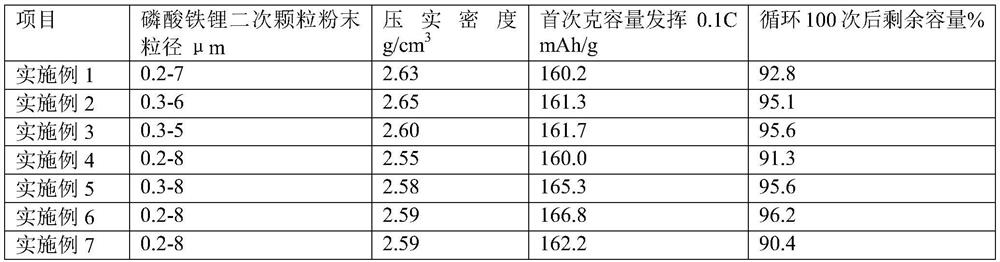
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com