Continuous synthesis method of metronidazole
A technology for metronidazole and chemical synthesis, applied in chemical instruments and methods, organic chemistry, chemical/physical processes, etc., can solve the problems of inaccurate feed amount, easy generation of impurities, high impurity content, etc., to achieve increased reaction temperature, The effect of convenient feeding and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The invention provides a continuous synthesis method of metronidazole, which comprises the following reactions:
[0026] S1. Synthesis of 2-methylimidazole.
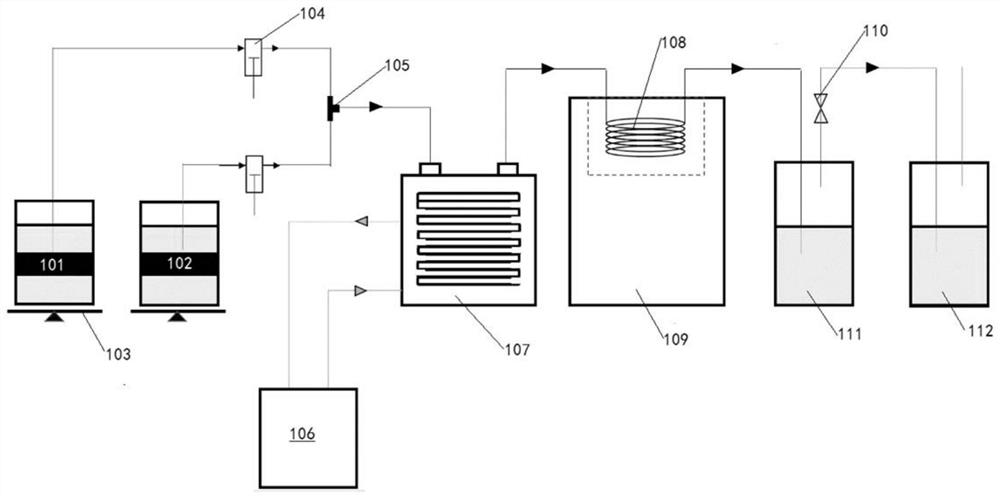
[0027] For the flow chart of the synthesis equipment, please refer to figure 1 :
[0028] After mixing the aldehyde mixture of glyoxal and acetaldehyde and ammonia water, it is pre-cooled to 10-20 ℃, and then enters the SK type tubular reactor to react at 1.8-2.0 Mpa, and the temperature of the reaction liquid is 55-60 ℃; The time is 18-22min; after the reaction is completed, the temperature is lowered to 10-15°C, and then concentrated, the concentration temperature is 58-62°C, and the concentration time is 1-3h, followed by crystallization; 2-methylimidazole is obtained by filtration and washing.
[0029] Specifically, in the present application, acetaldehyde and glyoxal are pre-mixed at a temperature below 20° C. and then placed in the aldehyde water bucket 102 . They are respectively sent to the jacket mixer...
Embodiment 1
[0063] Example 1: Synthesis of 2-methylimidazole.
[0064] After pre-mixing 10kg of 40% glyoxal and 9.1kg of 40% acetaldehyde below 20°C, they were sent to the jacket mixer with the aqueous ammonia solution through the feed pump, pre-cooled to 15°C, and then entered into the fan-tooth mixer. , after mixing again, enter the first tubular reactor 107 of Φ8 50m built-in SK type to carry out the reaction at 2.0Mpa, the oil temperature of the first tubular reactor 107 is controlled at 55 ° C, the temperature of the reaction liquid is controlled at 55 ° C, the reaction The time is 18-22min. When the reaction solution flows out from the first tubular reactor 107, after temperature sensing (monitoring the reaction temperature at any time), it enters the cooling pipe 108, is cooled to 15 ° C, and the reaction solution is concentrated under reduced pressure, and the concentrated temperature is 60 ° C, and the concentrated The time was 2h, cooling, crystallization, pressure filtration, ...
Embodiment 2
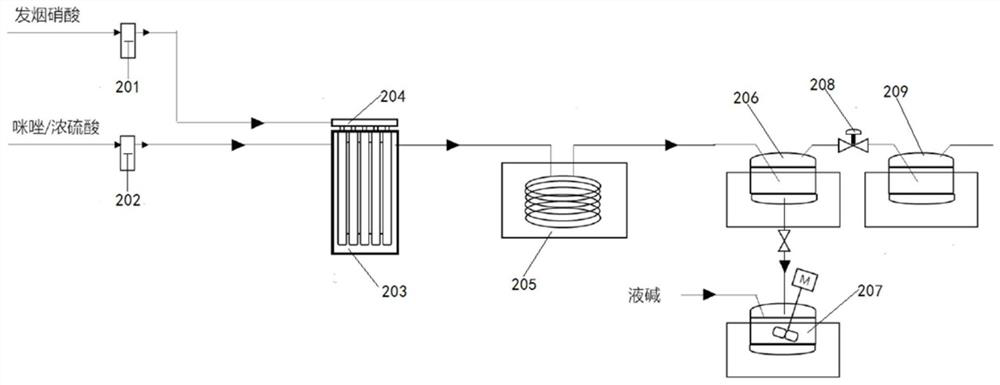
[0065] Example 2: Synthesis of 2-methyl-5-nitroimidazole by nitration reaction
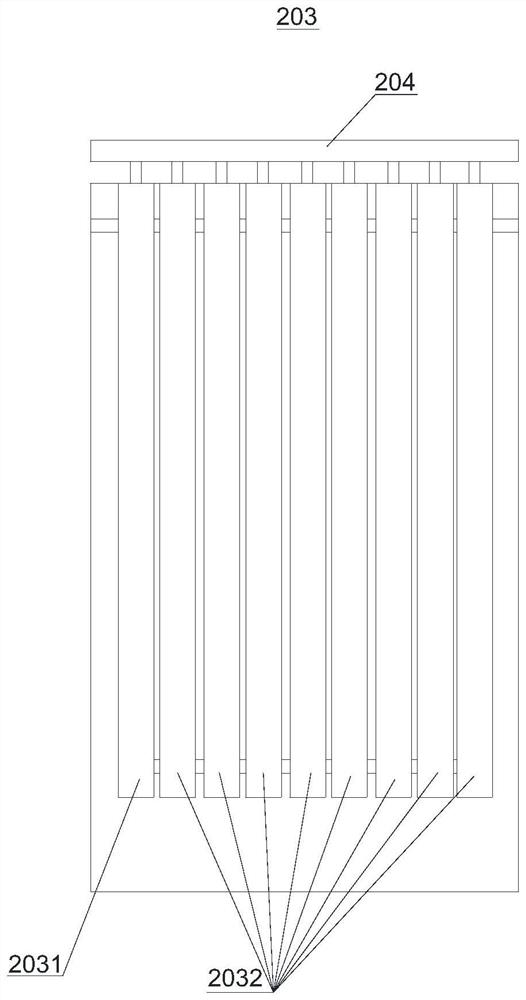
[0066] The imidazole sulfuric acid solution that is mixed with 2-methylimidazole and sulfuric acid is sent into the second reaction preheating channel 2031 with a flow rate of 20g / min, and the nitric acid is divided into 10 strands and enters 10 second reaction channels 2032 with a flow rate of 15g / min respectively ( The inner temperature is 120-140°C), the temperature of the imidazole sulfuric acid solution in the second reaction preheating channel 2031 reaches 110°C and then enters the first second reaction channel 2032 to mix with the first nitric acid, and the reaction mixture flows out of the first second reaction channel 2032. After the second reaction channel 2032, enter the second second reaction channel 2032 and the second nitric acid to mix and react, and so on, until the reaction mixture flows out from the last second reaction channel 2032, and the reaction mixture is in the nitrificatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com