Glucose and H2O2 dual-response double-layer cross-linked polymer nano drug delivery system as well as preparation method and application thereof
A nano-drug delivery system and cross-linked polymer technology, applied in biochemical equipment and methods, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problem of weakening long-term circulation, hypoglycemia, low drug loading, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
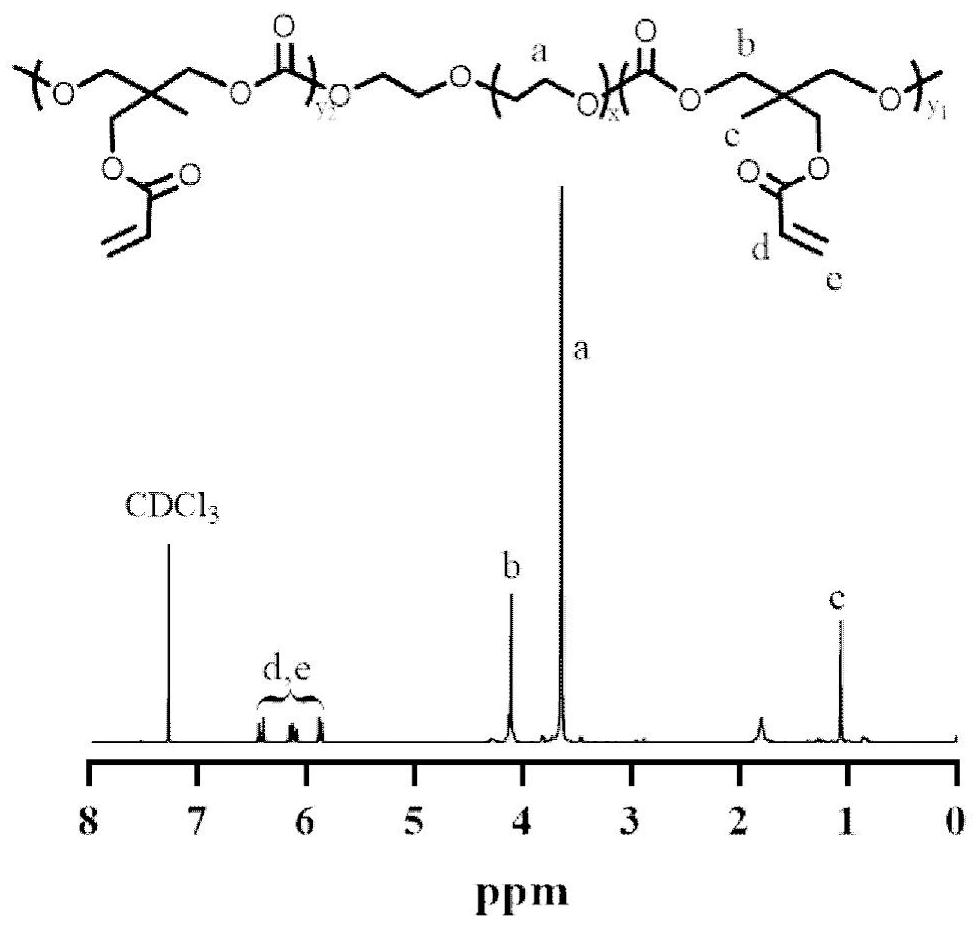
[0040] The synthesis of PEG-b-PAC and PEG-b-P (AC-co-MPA), the synthetic route and process are as follows:
[0041]
[0042] In a dry glove box under nitrogen protection, 0.67g polyethylene glycol (PEG, 8000Da, 0.084mmol) and 0.5g acryloyl carbonate (AC, 2.5mmol) were added to the sealed reaction flask, and 4mL of dichloromethane was added to dissolve After that, 50 mg of bis(bistrimethylsilyl)amine zinc (Zn[N(SiMe 3 )i] 2 ) (0.13 mmol). The reaction vessel was sealed and placed in an oil bath, and stirred at 45 °C for 72 h. After the polymerization reaction, the solution was dropped into ice ether for precipitation, and dried in vacuo to obtain 0.97 g of white solid powder PEG-b-PAC with a yield of 83%. . Take 1.18g PEG-b-PAC (0.1mmol), 0.148mg mercaptopropionic acid (MPA, 1.4mmol), 100μL Et 3 N (0.72 mmol) was dissolved in 10 mL of DMF, and the carbon-carbon double bond and thiol molar ratio was 4:3 at room temperature for overnight reaction, and the white solid PEG-P...
Embodiment 2
[0046] The synthesis of PEG-b-P (AC-co-MAPBA), the synthetic route and the process are as follows:
[0047]
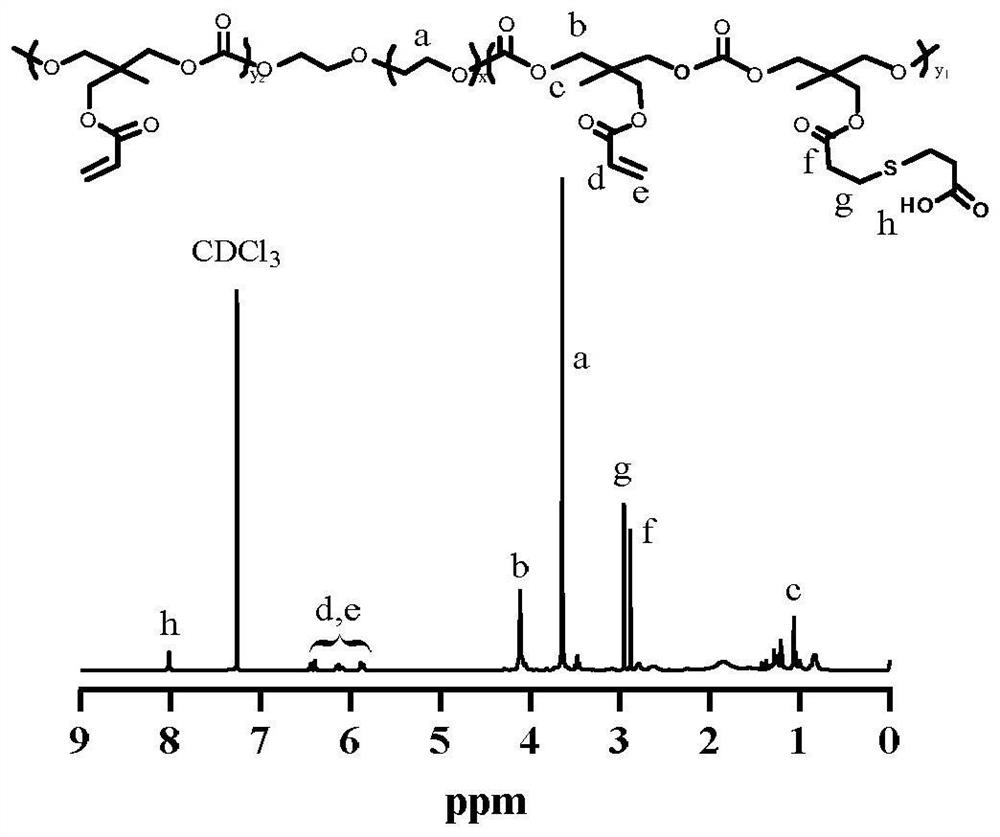
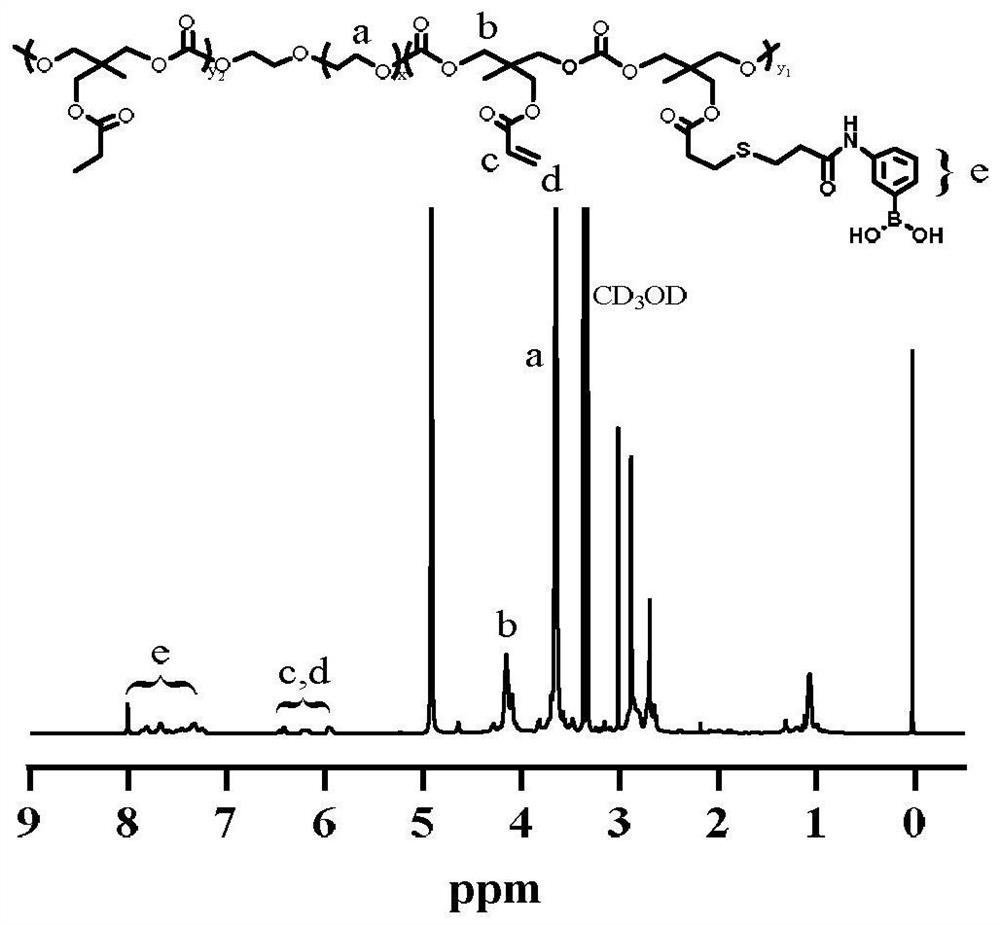
[0048] Accurately weigh 0.876g PEG-b-P(AC-co-MPA) (0.067mmol) and dissolve it in 10mL N,N-dimethylformamide (DMF), add 0.46g EDC·HCl (2.4mmol) and 0.276g NHS (2.4mmol) After activation, the reaction was stirred for 30 min, and then 0.50 g of m-aminophenylboronic acid (APBA, 3.2 mmol) was added to continue the reaction overnight. Dialysis was performed in methanol for 12 h with a dialysis bag with a molecular weight cut-off (MWCO) of 3500 Da. Methanol was removed by vacuum rotary evaporation. The solution was dialyzed against water and dried in vacuo to give the product PEG-b-P(AC-co-MAPBA).
[0049] H NMR characterization of PEG-b-P(AC-co-MAPBA) is attached image 3 , 1H NMR (400MHz, MeOD)δ1.05(s, 3H), 2.39-3.14(m, 3H), 2.83-3.09(m, 4H), 3.63(s, 52H), 3.95-4.35(m, 6H) , 5.56-6.71 (m, 1H), 7.13-8.05 (m, 3H).
Embodiment 3
[0051] The synthesis, synthetic route and process of GOx-MAA-MPD are as follows:
[0052]
[0053] 8 μL of α-methacrylic acid (MAA) was added to 400 μL of DMF, and then EDC·HCl and NHS were added sequentially. After half an hour, 3 mL of PB (pH 7.4, 5 mmol) containing 12 mg of GOx was added. The mixture was dialyzed against MWCO 3500Da dialysis bag for 24 h in deionized water to remove unreacted MAA. Vacuum drying gave solid GOx-MAA. Subsequently, GOx-MAA was treated with 1-thioglycerol (MPD) and triethylamine (Et 3 N) Reaction for 12h. The solution was dialyzed for 24 h and dried under vacuum.
[0054] Hydrogen NMR characterization of GOx-MAA-MPD is attached Figure 4 , 1 H NMR (400MHz, D 2 O)δ1.08(t, 1H), 1.29(t, 3H), 2.37-2.49(m, 1H), 2.65(s, 1H), 2.87(s, 2H), 3.07-3.28(m, 4H), 3.71(s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com