Phytase mutant and preparation method thereof
A technology of mutants and phytase, applied in the field of phytase mutants and its preparation, can solve problems such as strategies that have not been adopted for transformation, and achieve the effects of improving catalytic efficiency, improving catalytic efficiency, and enhancing pH tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Wild phytase PhyAn is derived from Aspergillus neoniger , belonging to the HAP family, the conserved amino acid sequence of the phytase catalytic region of this family is RHGX 1 RX 2 P, where X 1 and X 2 It is a variable amino acid residue, that is, phytases of the HAP family with the same conserved catalytic site will produce differences in these two amino acids, and the catalytic region sequence of phytase PhyAn is RHGARYP.
[0031] The invention aims at improving the catalytic efficiency and pH stability of the wild phytase PhyAn, and rationally designs its amino acid sequence to construct a mutant.
[0032] The catalytic efficiency is related to the binding affinity of the enzyme to the substrate, and then is closely related to the steric hindrance of the amino acid side chain in the catalytic region; secondly, the pH tolerance of the enzyme is inseparable from the charge of the amino acid, and the enzyme plays a key role in the activity of the charged part. Ami...
Embodiment 2
[0037] Example 2: Expression of phytase PhyAn and its mutant A63G+Y65H in Pichia pastoris GS115.
[0038] (1) Phytase PhyAn and its mutant A63G+Y65H were transformed into Pichia GS115;
[0039] The expression vectors of phytase PhyAn and its mutant A63G+Y65H were extracted with Plasmid DNA Kit and linearized with Sac1 restriction endonuclease. The purified products will be transformed . Transfer 10 μL of the enzyme digestion product to be transformed into 100 μL of Pichia GS115 competent cells, and transfer the above mixed samples to a 2 mm electroporation cup and incubate on ice for 15 min at a working voltage of 2 kV and pulse time. The electric shock was performed under the condition of about 0.5ms. Immediately after the end, 1 mL of pre-cooled 1 M sorbitol solution was added. After mixing, it was transferred to a new 1.5 mL centrifuge tube and placed in a 30 °C incubator for 1 incubation. h, spread in YPD medium after centrifugation, and cultured at 30 °C for 3-5 days. ...
Embodiment 3
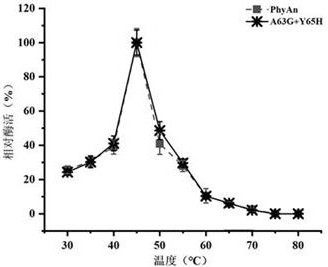
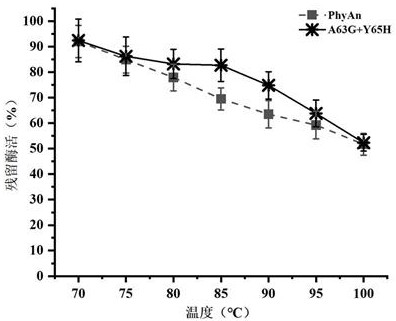
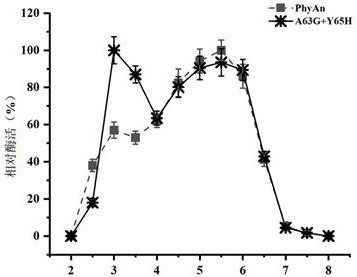
[0046] Analysis of Enzymatic Properties of Recombinant Phytase PhyAn and Its Mutant A63G+Y65H
[0047] The activity detection of phytase was carried out according to the molybdenum vanadium method given in the national standard of the People's Republic of China "GB / T 18634-2009". The specific determination method is as follows: dilute the phytase enzyme solution with 0.25 mol / L acetic acid buffer at pH 5.5 to an appropriate multiple, take 0.2 mL of the diluted enzyme solution into a 25 mL test tube, add 1.8 mL of acetic acid buffer, and vortex to mix After homogenization, the test tube was placed in a 37 °C water bath to preheat for 5 min; then 4 mL of 7.5 mmol / L sodium phytate solution was added to the test tube, and after mixing, the mixed sample was placed in a 37 °C water bath for 30 min. Add 4 mL of stop solution to the test tube, mix by vortex and let stand for 10 min at room temperature, and measure its absorbance at 415 nm wavelength.
[0048] The enzymatic activity o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com