Fluopyram synthesis method
A technology of fluopyram and a synthesis method, which is applied in the field of chemical synthesis, can solve the problems of prolonged process flow, low yield, complicated hydrogenation reaction, etc., and achieves cheap and easy availability of raw materials, improved reaction yield, and enhanced mass transfer. effect of the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
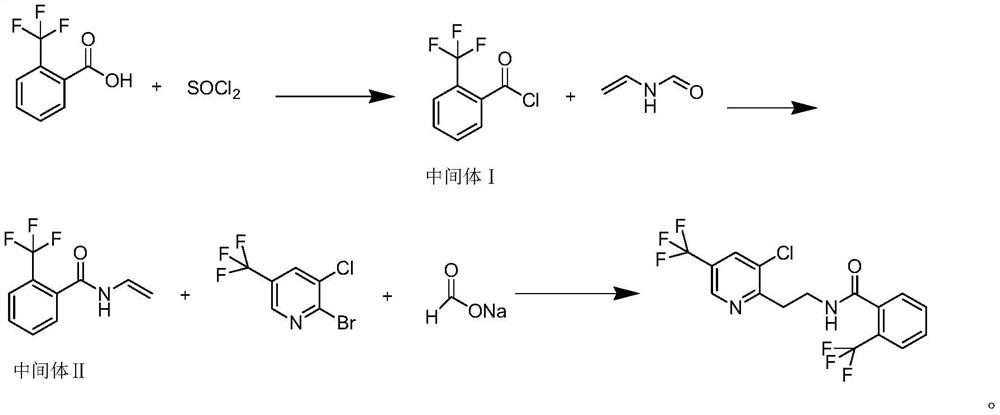
[0039] A method for synthesizing fluopyram, comprising the following steps:
[0040] Step A: According to the molar mass and volume ratio of 2-trifluoromethylbenzoic acid, thionyl chloride and reaction solvent A, 1mol:1mol:1L, add 190.12g of 2-trifluoromethylbenzoic acid to a 2L there-necked flask, and react Solvent A 1 L of 1,2 dichloroethane, stir and mix at 15 ° C, then slowly add 118.97 g of thionyl chloride, heat up to 60 ° C, keep the reaction for 3 hours, control the reaction solution, detect the raw material ≤ 0.2%, and then at - 0.098mP was desolvated under reduced pressure to 85°C, and the reaction solvent A and the remaining thionyl chloride were removed to obtain 206.45g of intermediate I 2-trifluoromethylbenzoyl chloride, with a content of 99% and a single-step yield of 98%;
[0041] Step B: Under nitrogen protection, after mixing 71.08 g of N-vinylformamide with 500 ml of dry THF, slowly add 120 g of acid binding agent triethylamine, then cool the reaction soluti...
Embodiment 2
[0044] A synthetic method of fluopyram, comprising the following steps:
[0045] Step A: same as Example 1, to obtain Intermediate I 2-trifluoromethylbenzoyl chloride 205.9g, the content is 99%, and the single-step yield is 98%;
[0046] Step B: same as Example 1, obtain 210.87 g of intermediate IIN-vinyl-2-(trifluoromethyl)benzamide, content 94%, single-step yield 94%;
[0047] Step C: Intermediate II N-vinyl-2-(trifluoromethyl)benzamide 210.87g, 2-bromo-3-chloro-5-(trifluoromethyl)pyridine 280g, sodium formate 68.01g, catalyst A 0.5 g of 10-phenylphenothiazine and 0.1 g of catalyst B cyclohexanethiol were added to 1 L of reaction solvent C dimethyl sulfoxide (purity 95%) at the same time. After stirring and dissolving, at room temperature, pumped continuously In the borosilicate glass microreactor, turn on the blue light, set the wavelength band to 420, adjust the pressure in the reactor to 2MP, set the reaction flow time to 45s, and in the borosilicate glass microreactor, ...
Embodiment 3
[0049] A method for synthesizing fluopyram, comprising the following steps:
[0050] Step A: Same as Example 1; 206.3 g of intermediate I 2-trifluoromethylbenzoyl chloride was obtained, with a content of 99% and a single-step yield of 98%;
[0051] Step B: same as Example 1; Intermediate IIN-vinyl-2-(trifluoromethyl)benzamide 211.77g, content 93.6%, single-step yield 94%;
[0052] Step C: Intermediate II N-vinyl-2-(trifluoromethyl)benzamide 211.77g, 2-bromo-3-chloro-5-(trifluoromethyl)pyridine 280g, sodium formate 68.01g, catalyst A 0.5 g of 10-phenylphenothiazine and 0.1 g of catalyst B cyclohexanethiol were added to 1 L of reaction solvent C dimethyl sulfoxide (purity 95%) at the same time. After stirring and dissolving, at room temperature, pumped continuously In the borosilicate glass microreactor, turn on the blue light, set the wavelength band to 420, adjust the pressure in the reactor to 1MP, set the reaction flow time to 30s, and pass the pyridyl halide radical in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com