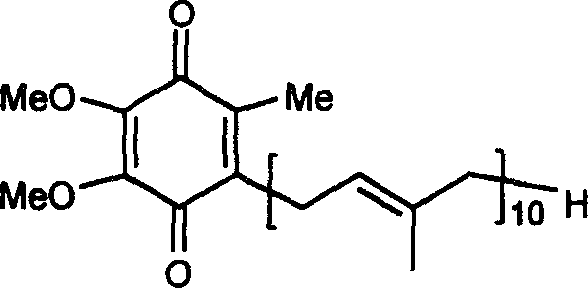
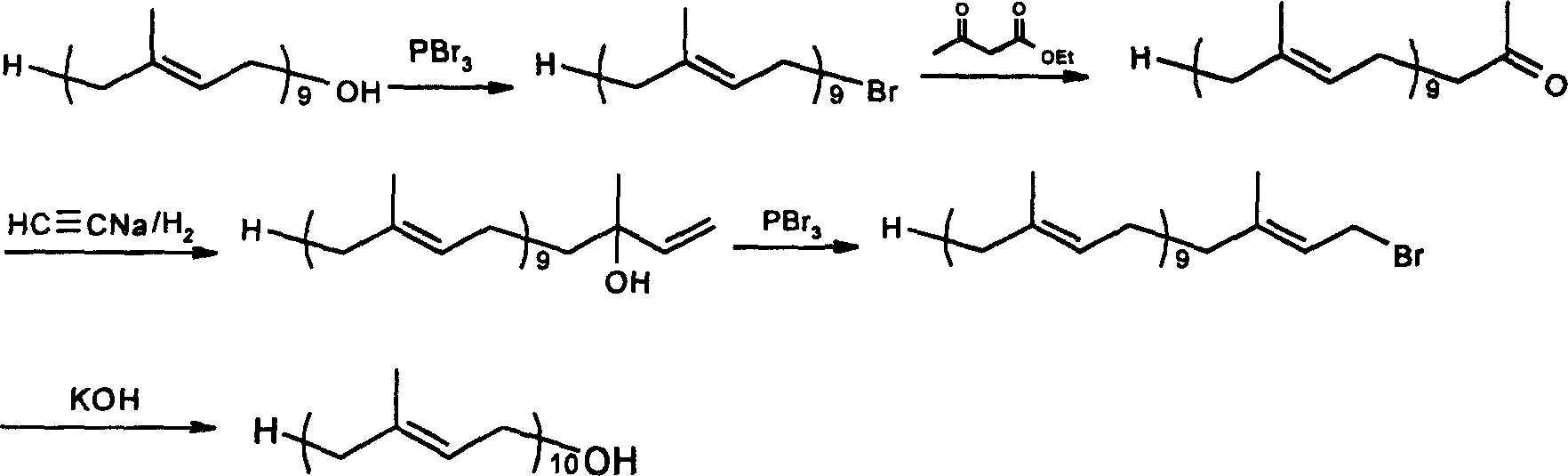
Method for synthesizing deca-isoprene alcohol
A technology of decyl isoprenol and synthesis method, which is applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve problems such as inability to realize industrialized production, limitation of large-scale application, active chemical properties, etc. To achieve the effect of reducing difficulty and workload, stable nature and easy storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of 5-Sulfonyl Decaprenol Acetate
[0034]
[0035] Dissolve 0.75g of solanyl sulfone in 10ml of tetrahydrofuran and 2ml of dimethylformamide in a mixed solution, cool in an ice-salt bath to -15°C, add 0.56g of potassium tert-butoxide under vigorous stirring, stir for 10-15 minutes, add 4- 0.20 g of chloro-3-methyl-2-buten-1-ol acetate and 0.20 g of sodium iodide were stirred for 15 hours, during which the temperature of the system rose from -15°C to 10°C. Stop the reaction, add 50ml of ether, wash with 5% phosphoric acid, wash with water, wash with saturated brine, and remove the solvent to obtain 0.74g of light yellow oil, with a yield of 85%. 1 H-NMR analysis confirmed that it was 5-sulfonyl deprenol acetate.
Embodiment 2
[0045] Synthesis of Decaprenol
[0046]
[0047] 0.88g of 5-sulfonyldecaprenol acetate was dissolved in anhydrous ether and added to a 100ml three-neck flask. The system was cooled to -65°C, and 25ml of liquid methylamine was added. 0.14g of lithium ribbon was cut and put into the reaction system, and stirred vigorously for a period of time. The reaction was quenched by adding isoprene, methanol and ammonium chloride. After rising to room temperature, the reaction solution was poured into ice water containing diethyl ether, separated, the aqueous phase was extracted with diethyl ether, and the organic phases were combined. After treatment, 0.60 g of a light yellow oily product was obtained, with a yield of 86%. product by 1 H-NMR confirms that it is deprenol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com