Conjugate derivative material of 9-phenyl-9-pyrenyl fluorene substituted pyrene its preparation method and application
A kind of derivative, the technology of pyrenyl fluorene, which is applied to the conjugated derivative material of pyrene substituted by 9-phenyl-9-pyrenyl fluorene and its preparation and application fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1: Synthesis of 2-pyrenyl-9-phenyl-9-pyrenylfluorene (2P9PPF) (Compound I).
[0074] (1) Synthesis of 2-bromo-9-phenylfluoren-9-ol (2-Bromo-9-phenyl-fluoren-9-ol)
[0075] Use magnesium scraps (0.58g, 24mmol), a small amount of iodine and bromine benzyl ((4.48g, 29mmol) in anhydrous ether (or anhydrous tetrahydrofuran) (30mL) to make phenylmagnesium bromide Grignard reagent, the reagent used Dilute with 20mL of anhydrous ether, and then add anhydrous tetrahydrofuran dissolved with 2-bromofluorenone (3.77g, 14.6mmol) dropwise into Grignard reagent, stir for 4h, add saturated ammonium chloride solution for hydrogenolysis after cooling for 2h. The reaction mixture Extract twice with dichloromethane, wash with water, then dry with anhydrous magnesium sulfate.Use ethyl acetate / petroleum ether=10:1 as eluent column chromatography on silica gel to obtain light yellow solid (4.65g, 13.8mmol ), yield 94%). 1 HNMR (400MHz, CDCl3) δ (ppm): 7.65 (d, J = 8.0Hz, 1H); 7.44-7...
Embodiment 2
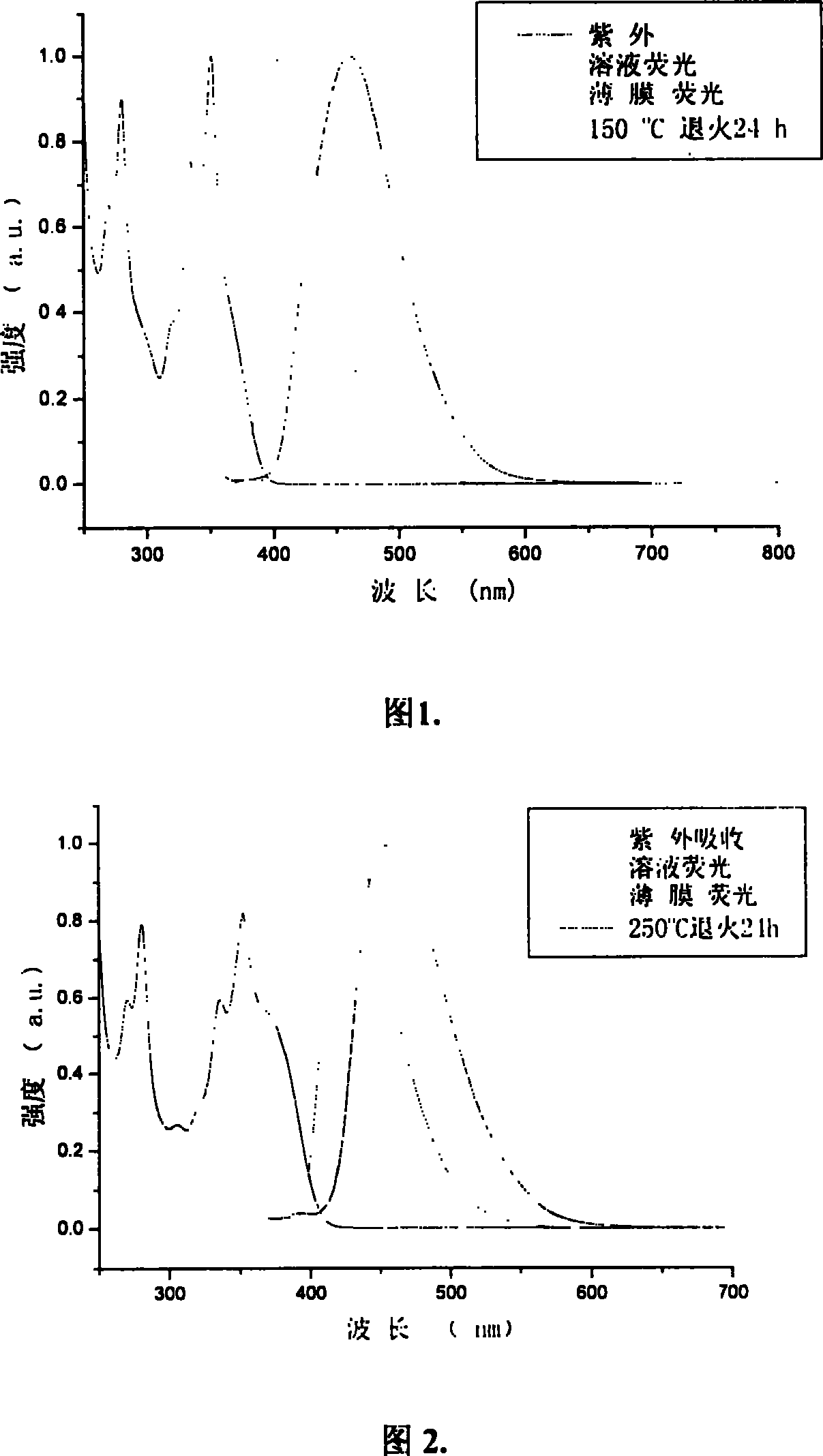
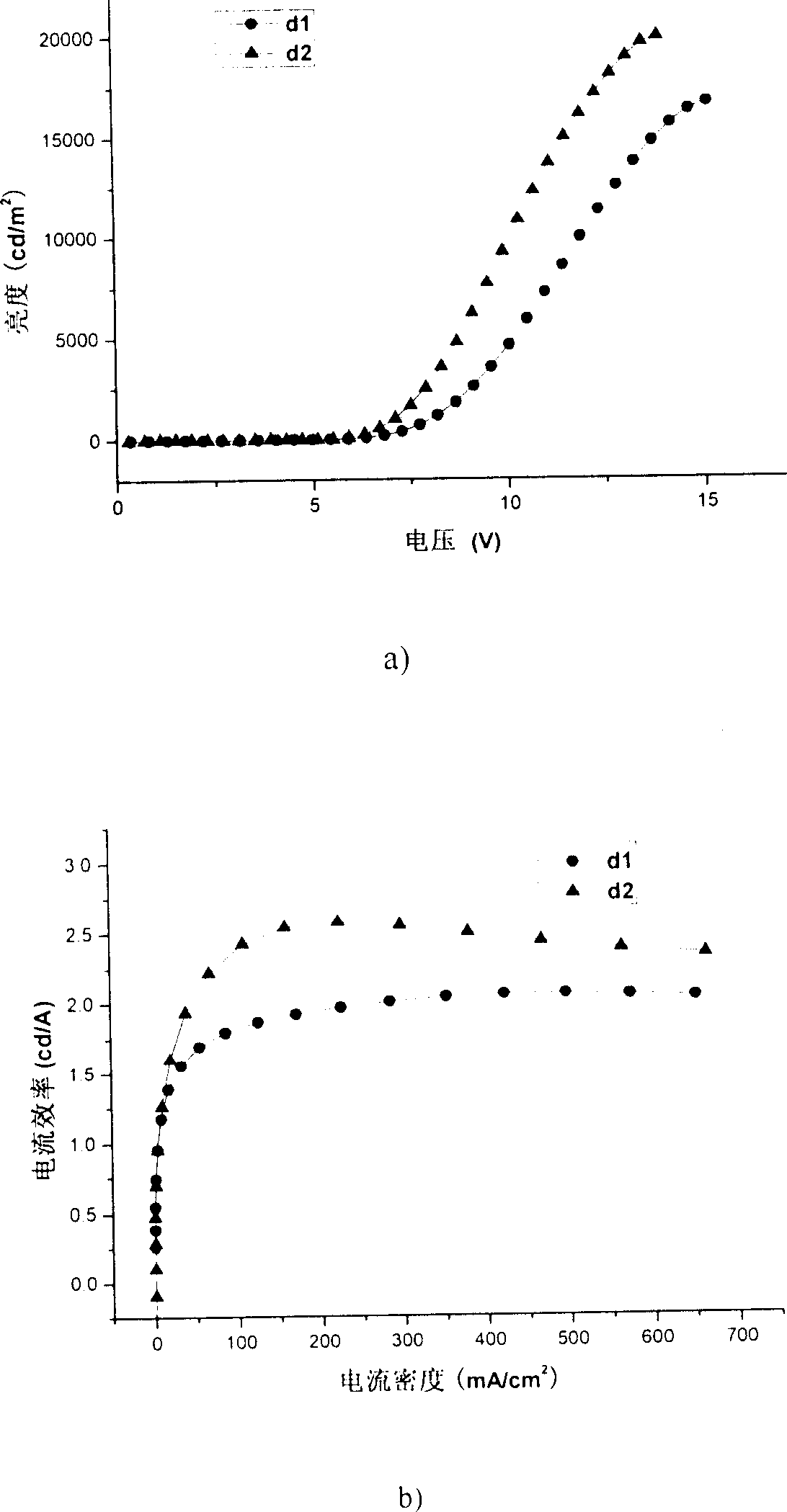
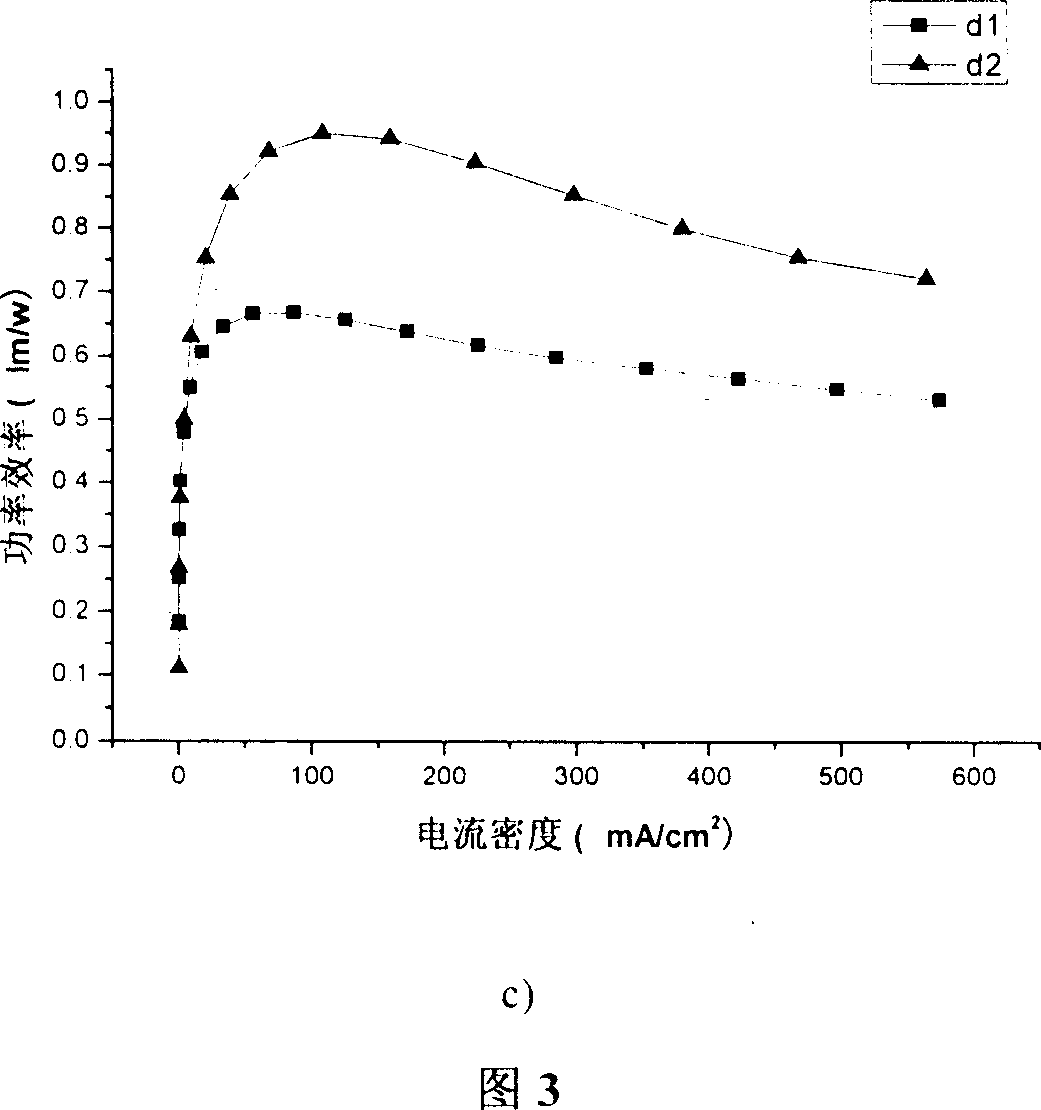
[0085] Embodiment 2: to the ultraviolet absorption spectrum of the trimer 2P9PPF (product in embodiment 1) containing anthracene and pyrene fluorene in 9 positions, photoluminescence spectrum, spectral thermal stability and quantum efficiency measurement:
[0086] Dissolve 2P9PPF in dichloromethane dilute solution, and use Shimadzu UV-3150 ultraviolet-visible spectrometer and RF-530XPC fluorescence spectrometer to measure the absorption and emission spectra. The photoluminescence spectrum was measured at the maximum absorption wavelength (351 nm) of ultraviolet absorption. The solid film is formed by dropping the solution on a transparent glass plate after the solvent evaporates. The fluorescence quantum efficiency of the solution is measured by 10 in cyclohexane -6 A 9,10-diphenylanthracene solution of M (quantum efficiency 0.9) was used as a standard for measurement.
[0087] The maximum absorption peak of the 2P9PPF solution at greater than 300nm is 351nm, and the maximum...
Embodiment 3
[0089] Example 3: Synthesis and spectrometry of 2,7-dipyrenyl-9-phenyl-9-pyrenylfluorene (DPPPF) (compound II).
[0090] The synthetic method similar to 2P9PPF can synthesize 2,7-dipyrenyl-9-phenyl-9-pyrenylfluorene (DPPPF), but 2,7-dibromofluorene is used for fluorene, and during the even chain reaction, 1-(4 , the amount of 4,5,5-tetramethyl-1,3,2-dioxoboronate 2-yl)pyrene should be greater than that of 2,7-dibromo-9-phenyl-9-pyrenylfluorene double.
[0091] The NMR, MS and elemental analysis data of DPPPF are as follows. 1 H-NMR (400MHz, CDCl3) δ (ppm): 8.24-8.12 (m, 8H); 8.10-7.92 (m, 19H); 7.77 (dd, J=8.4Hz, 1.6Hz, 2H); 7.64 (d, 8.4Hz, 2H); 7.46(s, 2H); 7.34-7.28(s, 3H); 7.27-7.22(m, 2H). 13 C-NMR(400MHz,CDCl3)δ(ppm):152.80,140.92,139.04,137.75,131.63,131.06,130.76,130.62,129.27,128.56,128.48,127.85,127.60,126.98,126.57,126.18,125.37,125.28,125.04 , 124.98, 124.88.LDl-TOF-MS (m / z): Calcd.for C 67 h 38 842.3, found 842.2. Anal. Calcd. C, 95.46; H, 4.54; found C, 95....
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com