Stainless steel cardiovascular bracket with pharmaceutical coating on surface and preparation method thereof
A drug coating and cardiovascular technology, which is used in drug devices, medical preparations containing active ingredients, blood vessels, etc., can solve the complex preparation process of heparinized polymerization, difficult to control the release rate of rapamycin, and difficult to meet clinical requirements. It can prevent the occurrence of subacute and long-term thrombosis, improve hydrophilicity and biocompatibility, and promote adhesion and growth.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
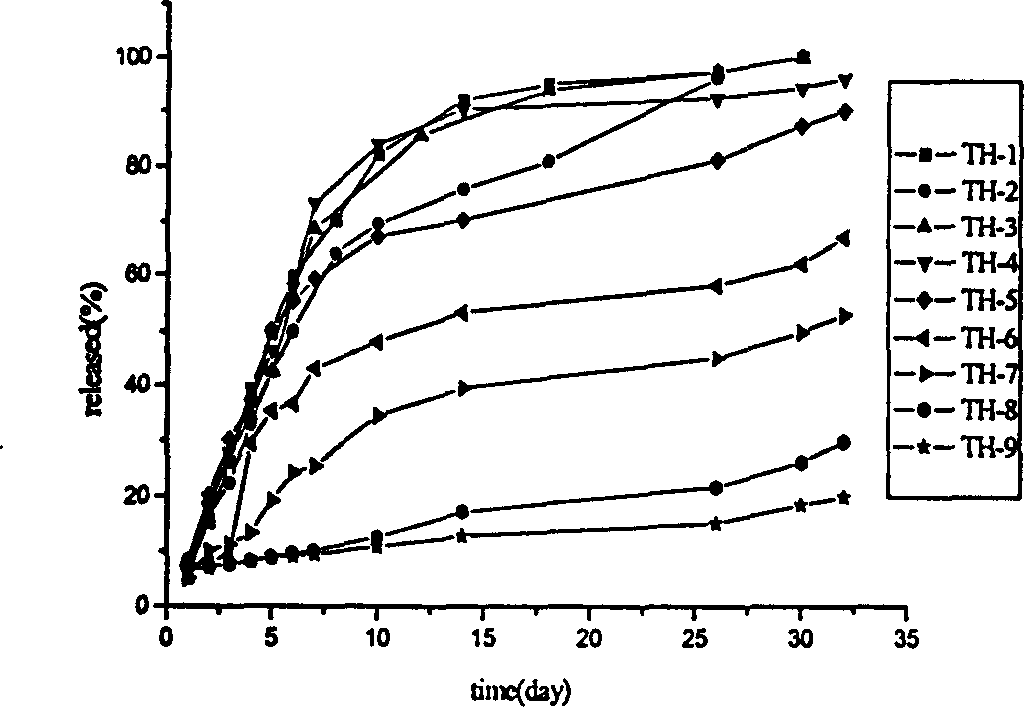
[0056] Take the 316L stainless steel bracket, wash and dry it. The above-mentioned stent was immersed in a solution containing 0.00004 g of paclitaxel, 0.40 g of ethylene-vinyl acetate, and 7.59 g of toluene for 30 seconds, taken out, and dried under vacuum at 60° C. for 20 hours. The stent was then immersed in a solution containing 0.2 g of ethylene-vinyl acetate and 9.8 g of toluene for 30 seconds, taken out and dried. Heparin grafting was initiated using a nitrogen plasma. The plasma treatment conditions are: RF power 20w, vacuum degree 50Pa, time 10min. Pass through 4% heparin aqueous solution for 30 minutes, and after drying, the surface heparin grafting amount is 5.3ug / cm 2 drug stent, which is denoted as TH-1. The polymer coating has a smooth, transparent surface and is firmly bonded to the substrate. Among them, the release rate of antiproliferative drugs is shown in the attached image 3 , after drug release, the amount of heparin on the surface of the stent was ...
Embodiment 2
[0058] Take the 316L stainless steel bracket, wash it, and dry it. The above-mentioned stent was immersed in a solution containing 0.02 g of paclitaxel, 0.18 g of ethylene-vinyl acetate, and 7.8 g of toluene for 100 seconds, taken out, and dried. Then dip the stent in a solution containing 0.1 g of ethylene-vinyl acetate and 9.9 g of toluene for 30 seconds, take it out, and dry it. Nitrogen plasma was used to initiate heparin grafting, and the plasma treatment conditions were: radio frequency power 100w, vacuum degree 10Pa, time 2min. Pass through 4% heparin aqueous solution for 10 minutes, and the grafted heparin amount after drying is 5.1ug / cm 2 The drug stent, the stent is recorded as TH-2. The polymer coating has a smooth, transparent surface and is firmly bonded to the substrate. Among them, the release rate of antiproliferative drugs is shown in the attached image 3 . After the drug release, the amount of residual heparin on the surface of the stent was 4.2ug / cm 2...
Embodiment 3
[0060] Take the 316L stainless steel bracket, wash and dry it. Spray 20 grams of a toluene solution containing 0.002 grams of paclitaxel and 0.198 grams of propylene-vinyl acetate on the surface of the stent and dry it. The stent was then dipped in a solution containing 0.2 g of ethylene-methyl methacrylate and 9.8 g of toluene, taken out, and dried. Heparin grafting was initiated using oxygen plasma. The plasma treatment conditions are: RF power 500w, vacuum degree 100Pa, time 10min. Pass through 4% heparin aqueous solution for 15 minutes, and the content after drying is 3.2ug / cm 2 The heparin drug stent, the stent is recorded as TH-3. The polymer coating has a smooth, transparent surface and is firmly bonded to the substrate. Among them, the release rate of antiproliferative drugs is shown in the attached image 3 . After the drug release, the amount of residual heparin on the surface of the stent was 2.8ug / cm 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com