One-step preparation process of aseptic ceftriaxone sodium for injection
A technology for ceftriaxone sodium and a preparation process, which is applied in the field of compound preparation, can solve the problems of large solvent usage, high production cost, high labor intensity and the like, and achieves less solvent usage, low production cost and low labor intensity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
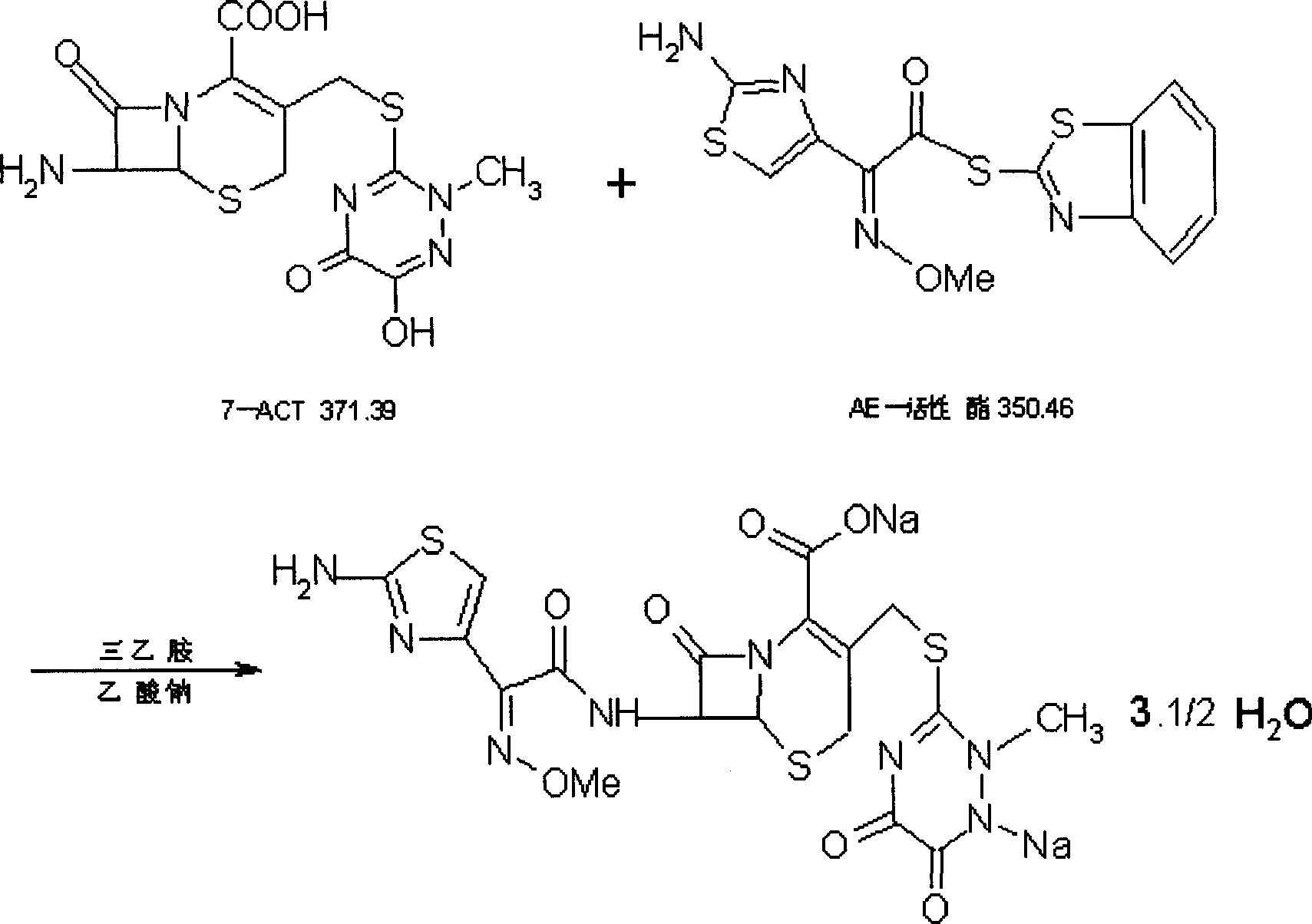
[0023] The preparation technology of ceftriaxone sodium of the present invention is as follows:
[0024] Add 50ml of dichloromethane, 20ml of ethanol, 18ml of triethylamine, and 5ml of water into a three-neck flask, stir and cool down under nitrogen protection; at a temperature of 5°C, add 20g of 7-ACT, 20g of AE-active ester, 0.5g of sodium bisulfite Oxygen agent; stirring reaction, temperature 6°C; reaction clarification, adding hydrochloric acid aqueous solution to adjust pH to 3.5, stirring for 30 minutes, adding 10 grams of sodium isooctanoate, layering, filtering; adding acetone insoluble organic solvent dropwise; solution slightly cloudy, adding crystals Seed and grow crystals; add acetone dropwise to crystallize at a temperature of 10°C; suction filter to obtain a crystal filter cake, wash with acetone; dry with hot air at 50°C; dry to obtain the product.
[0025] The dry product was 33.03g, and the yield was 92.78% (based on 7-ACT).
Embodiment 2
[0027] The preparation technology of ceftriaxone sodium of the present invention is as follows:
[0028] Add 50ml of dichloromethane, 20ml of ethanol, 18ml of triethylamine, and 5ml of water into a three-neck flask, stir and cool down under nitrogen protection; at a temperature of 5°C, add 20g of 7-ACT, 20g of AE-active ester, 0.5g of sodium bisulfite Oxygen agent; stirring reaction, temperature 7°C; reaction clarification, adding acetic acid aqueous solution to adjust pH to 4, stirring for 25 minutes, adding 10 grams of sodium acetate, layering, filtering; adding acetone insoluble organic solvent dropwise; the solution is slightly cloudy, add seed crystals Crystal growth; crystallization by adding acetone dropwise at a temperature of 10°C; suction filtering to obtain a crystallization filter cake, washing with acetone; drying with hot air at 50°C; drying to obtain the product.
[0029] The dry product was 33.01g, and the yield was 92.67% (based on 7-ACT).
Embodiment 3
[0031] The preparation technology of ceftriaxone sodium of the present invention is as follows:
[0032] Add 50ml of dichloromethane, 25ml of ethanol, 18ml of triethylamine, and 5ml of water into a three-neck flask, stir and cool down under nitrogen protection; at a temperature of 8°C, add 20g of 7-ACT, 20g of AE-active ester, 0.5g of sodium bisulfite Oxygen agent; stirring reaction, temperature 8°C; reaction clarification, adding hydrochloric acid aqueous solution to adjust PH to 3, stirring for 35 minutes, adding 10 grams of sodium isooctanoate, layering, filtering; adding isopropanol insoluble organic solvent dropwise; the solution is slightly cloudy, Add crystal seeds to grow crystals; add isopropanol dropwise to crystallize at 8°C; suction filter to obtain a crystallization filter cake, wash with isopropanol; dry with hot air at 50°C; dry to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com