Preparation method of injection type pH and glucose sensitive hydrogel
A glucose-sensitive, hydrogel technology, applied in non-active ingredient medical preparations, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve the problem of non-biodegradable, unfavorable ionic strength, biocompatibility of drug activity, Reduce biocompatibility and other problems, achieve the effect of maintaining activity, good biocompatibility and biodegradability, and simple and convenient preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] (1) Preparation of chitosan quaternary ammonium salt
[0032] Disperse chitosan and 2,3-epoxypropyltrimethylammonium chloride with a molar ratio of 1:2-1:8 in water or isopropanol, heat to a reaction temperature of 60-90°C, Reaction 2-12h. The reaction solution is poured into acetone for precipitation, washed, and dried to obtain chitosan quaternary ammonium salt.
[0033] The molecular weight range of chitosan is 5-2 million, and the degree of deacetylation is 65%-100%.
[0034] (2) Preparation of polyethylene glycol cross-linked chitosan quaternary ammonium hydrogel
[0035] The chitosan quaternary ammonium salt that above-mentioned step (1) gained is dissolved in the acidic solution of pH2.0-6.0 and obtains the chitosan quaternary ammonium salt solution of 1.0-10.0wt.%, and acidic solution comprises acetic acid, hydrochloric acid, lactic acid, Citric Acid, Ascorbic Acid, Formic Acid. Adding polyethylene glycol with a molecular weight range of 200-25000 in the chi...
Embodiment 1
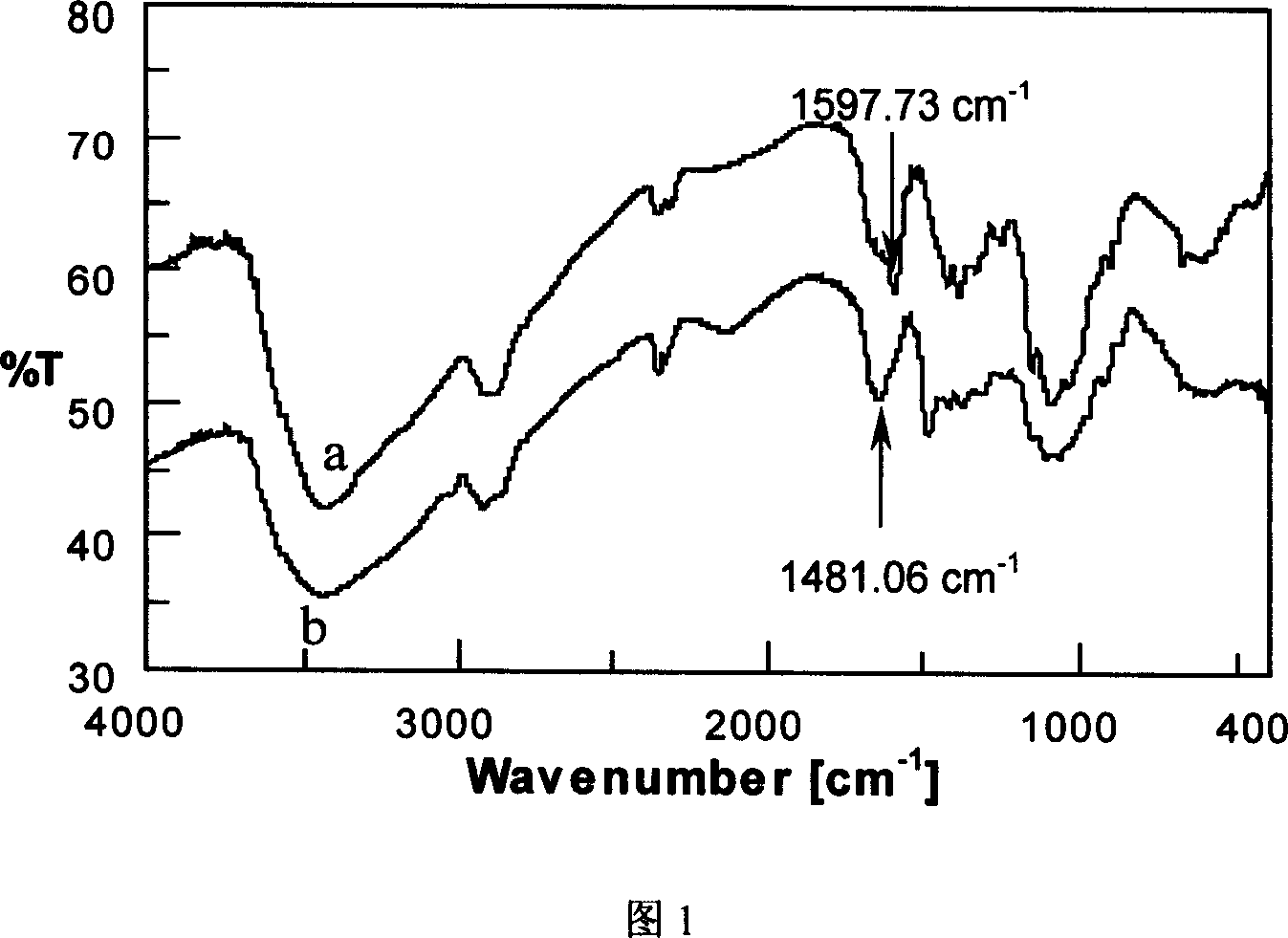
[0040] Disperse 3.0g of chitosan in 20mL of water and stir at 80°C for 1h. 16.94g of 2,3-epoxypropyltrimethylammonium chloride was dissolved in 40mL of water, and added to the chitosan dispersion. After stirring and reacting at 80° C. for 10 h, the reaction solution was poured into acetone to precipitate, stirred and washed overnight in a refrigerator, and then dried to obtain a white loose product. Characterized by infrared spectroscopy, see Figure 1, 1597cm after quaternization -1 The amino stretching vibration peak at 1481cm -1 appear-CH 3 The strong absorption peak of the C-H bending vibration of C-H proves that the quaternary ammonium group is substituted on the chitosan chain to generate the chitosan quaternary ammonium salt.
Embodiment 2
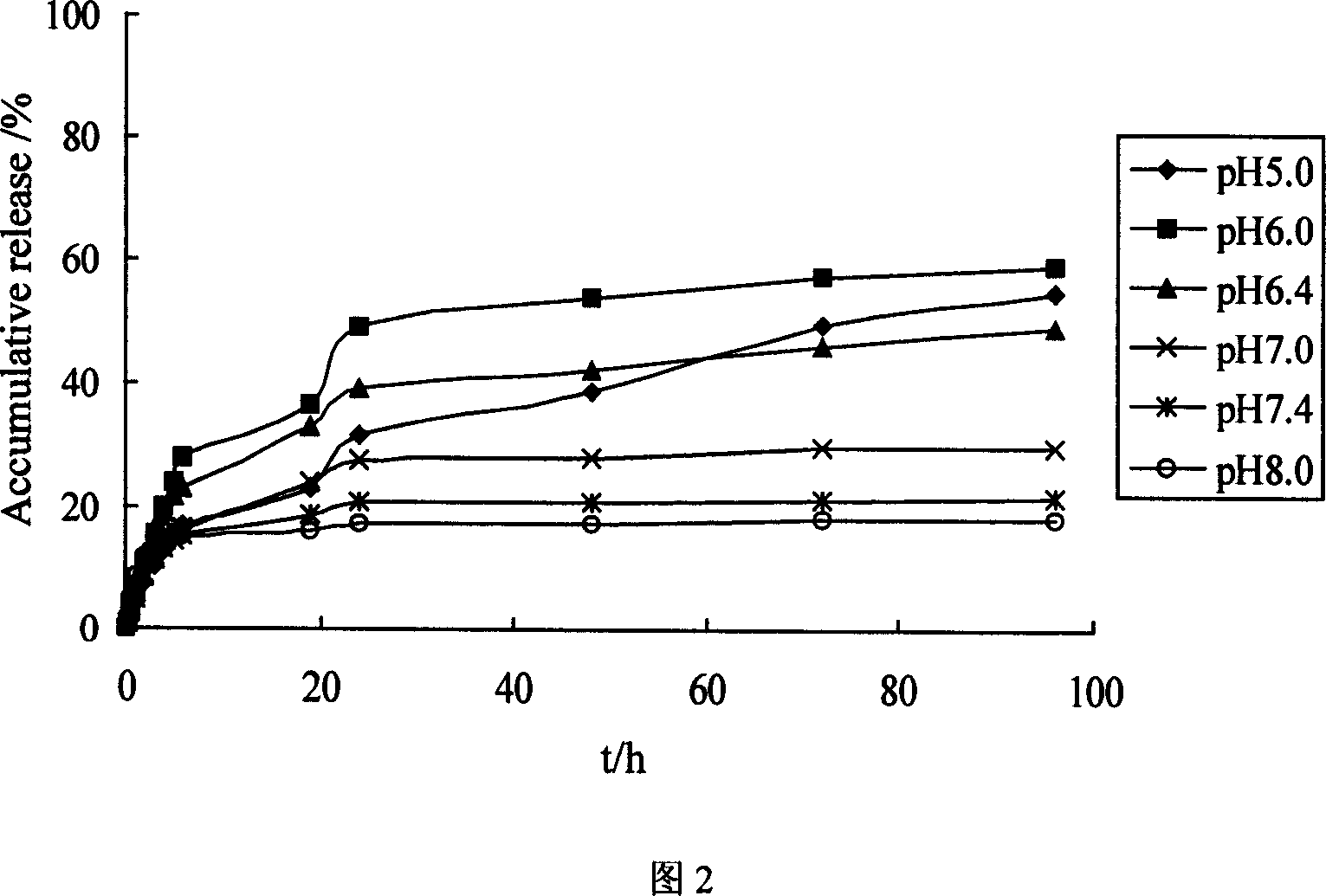
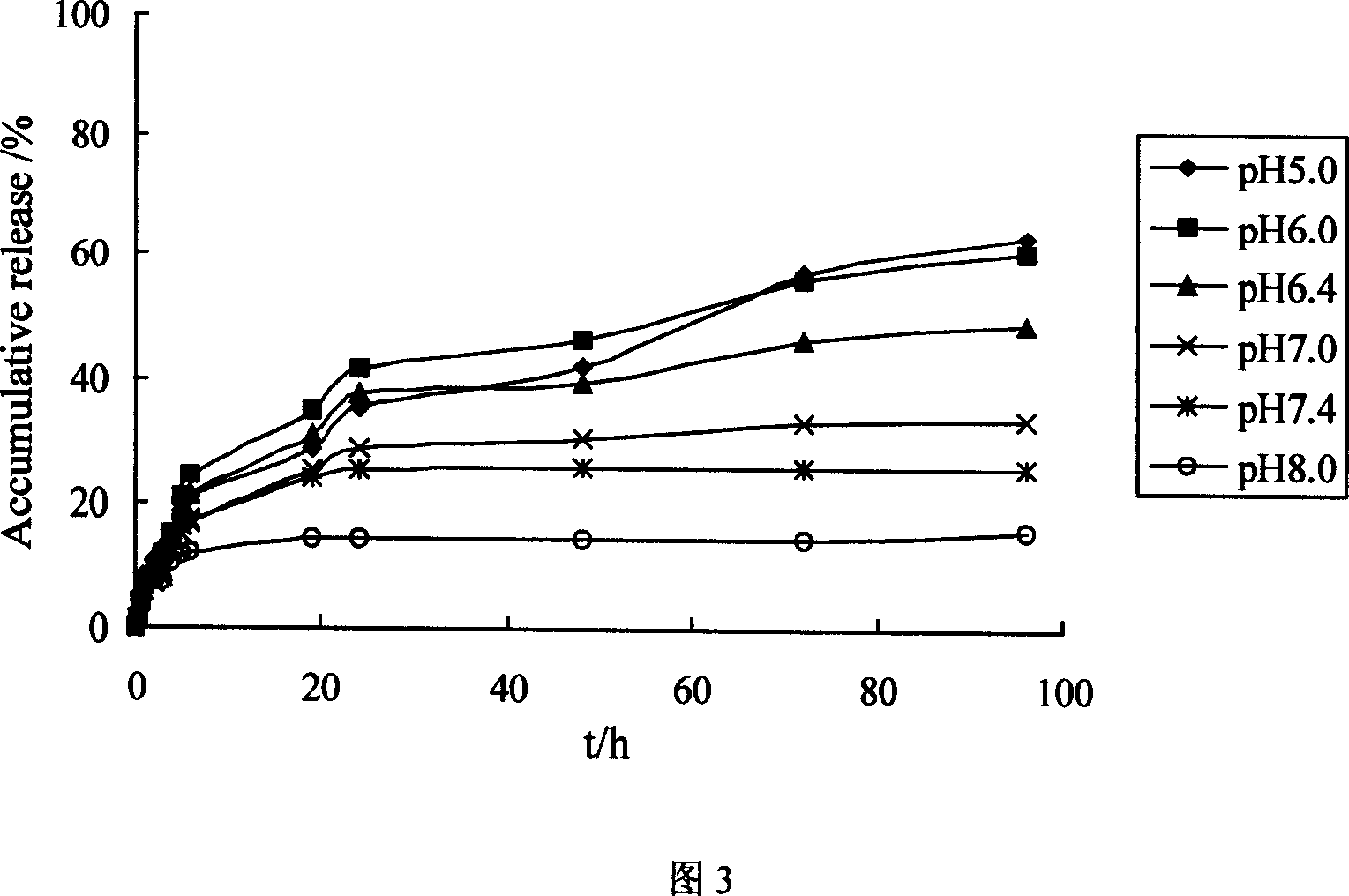
[0042] Dissolve 0.50 g of chitosan quaternary ammonium salt and 0.72 g of PEG8000 prepared in Example 1 in 8 mL of 0.1 mol / L lactic acid solution, add 2 mL of 0.125 g / mL beta-glycerophosphate sodium solution dropwise under stirring, and fully stir Mix it well. The pH of the resulting solution was 7.4. The mixed solution was heated to 37°C and kept for 60 minutes to fully gel the system. The system is stable at 4°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com