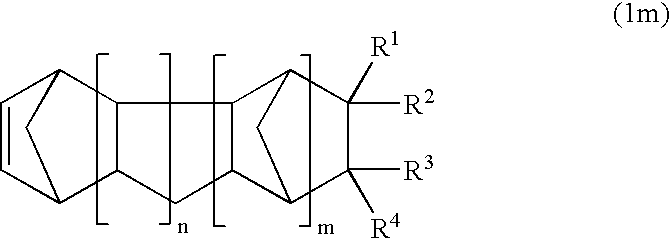
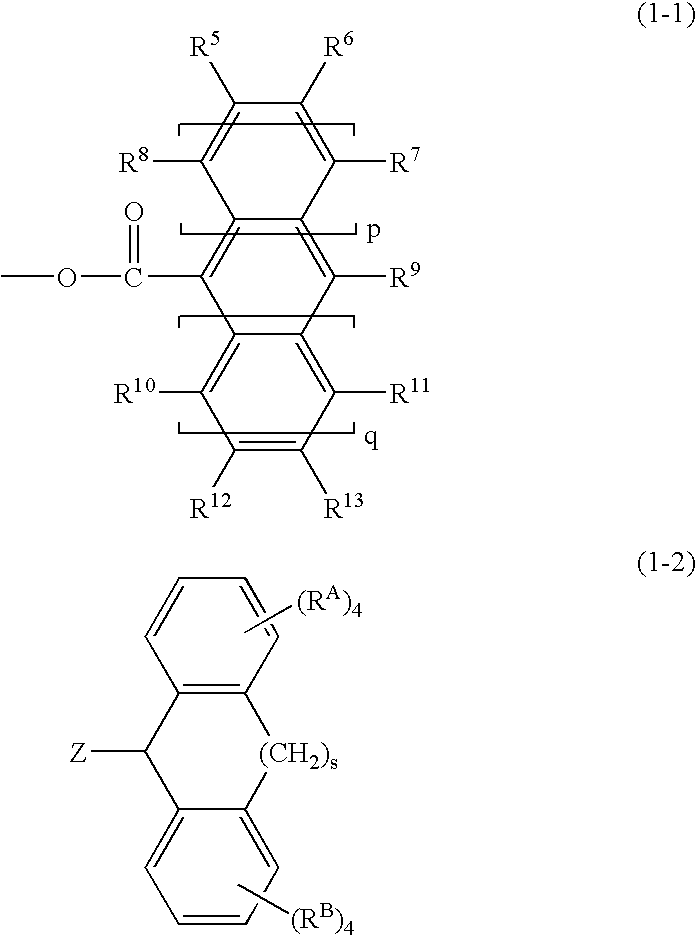
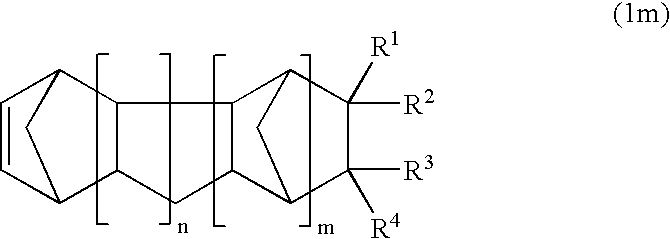
Norbornene derivative and norbornene polymer obtained therefrom through ring opening polymerization
a technology of norbornene and derivative, applied in the field of norbornene derivative, can solve the problems of heat resistance and water resistance, optical characteristics of the generated optical product also failed to meet the demands, etc., and achieve excellent transparency, improved light resistance, and improved workability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 5-(4-biphenylcarbonyloxy)bicyclo[2.2.1]hept-2-ene
[0291] 17
[0292] 28 g (253.9 mmol) of norbornene alcohol (endo form / exo form molar ratio of 8 / 1) was measured into a 500 mL flask equipped with a dropping funnel, and the air in the system was replace with nitrogen. 41 mL (507.8 mmol) of pyridine was then added dropwise, and stirred well with a stirrer to dissolve the pyridine. Subsequently, with the temperature of the reaction system maintained at 4.+-.2.degree. C. using an ice bath, and with adequate stirring, 50 g (230.8 mmol) of 4-phenylbenzoyl chloride dissolved in 200 mL of THF (tetrahydrofuran) was gradually added dropwise. Following completion of this dropwise addition, stirring was continued for 1 hour with the reaction system still in the ice bath, and then for a further 1 hour at room temperature, and finally the reaction system was refluxed for 30 minutes. Following cooling to room temperature, the generated pyridine salts were removed by filtration, and the re...
example 2
Synthesis of 5-(2-naphthalenecarbonyloxy)bicyclo[2.2.1]hept-2-ene
[0296] 18
[0297] With the exceptions of using 44 g (230.8 mmol) of 2-naphthoyl chloride instead of 4-phenylbenzoyl chloride, and purifying the reactants using a column (filler: Al.sub.2O.sub.3, developing solvent: hexane), reaction in the same manner as the example 1 yielded 46 g of a white solid of 5-(2-naphthalenecarbonyloxy)bicyclo[2.2.1]hept-2-ene. Analysis of the product monomer by HPLC revealed a purity of 99%.
[0298] Analysis of the .sup.1H-NMR spectrum in the same manner as described above for the example 1 revealed a stereoisomeric ratio (the endo type / exo type molar ratio) for the substituent group within the monomer of 8 / 1. Furthermore, in the infrared absorption (IR) spectrum, the stretching vibration absorption for the CH groups of the aromatic rings was observed within the vicinity of 3050 cm.sup.-1, and the stretching vibration absorption for the CO group within the ester carbonyl group was observed within...
example 3
Synthesis of 5-(1-naphthalenecarbonyloxy)bicyclo[2.2.1]hept-2-ene
[0300] 19
[0301] With the exception of using 44 g (230.8 mmol) of 1-naphthoyl chloride instead of 2-naphthoyl chloride, reaction in the same manner as the example 2 yielded 43.8 g of a transparent liquid of 5-(1-naphthalenecarbonyloxy)bicyclo[2.2.1]hept-2-ene. Analysis of the product monomer by HPLC revealed a purity of 98%.
[0302] Analysis of the .sup.1H-NMR spectrum in the same manner as described above for the example 1 revealed a stereoisomeric ratio (the endo type / exo type molar ratio) for the substituent group within the monomer of 8 / 1. Furthermore, in the infrared absorption (IR) spectrum, the stretching vibration absorption for the CH groups of the aromatic rings was observed within the vicinity of 3050 cm.sup.-1, and the stretching vibration absorption for the CO group within the ester carbonyl group was observed within the vicinity of 1710 to 1730 cm.sup.-1.
[0303] The .sup.1H-NMR spectrum and the infrared absor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com