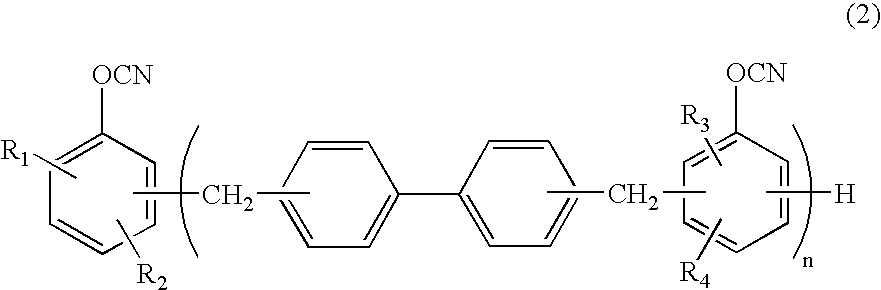
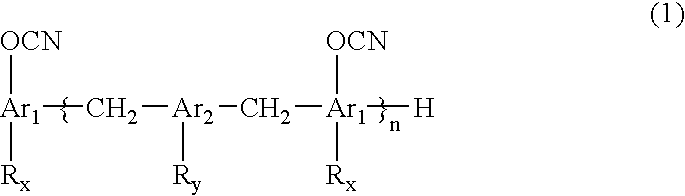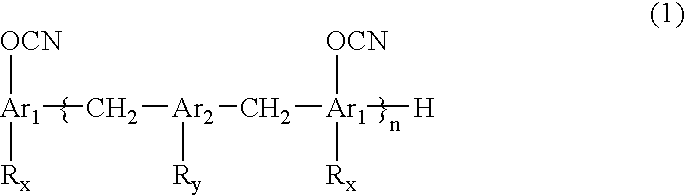Novel cyanate ester compound, flame-retardant resin composition, and cured product thereof
a technology of cyanate ester and resin, which is applied in the direction of insulating substrate metal adhesion improvement, instruments, water absorption, etc., can solve the problems of increasing physical properties required as a functional polymer material, increasing the number of times, and reducing the number of times. , to achieve the effect of low dielectric constant, excellent flame retardancy and low cos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example a1
Synthesis of Cyanate Ester of Biphenyl Novolak (Formula (9): to be Referred to as “G65C”)
[0057]
[0058] Biphenyl novolak having 1.1 mol of OH groups (supplied by Nippon Kayaku Co., Ltd., KAYAHARD-GPHG65) and 1.6 mol of triethylamine were dissolved in 900 ml of 3-methyltetrahydrofuran, to obtain a solution 1. The solution 1 was dropwise added to 2,500 g of a methylene chloride solution of 2.2 mol of cyanogen chloride at −10° C. over 1.5 hours. The mixture was stirred for 30 minutes. Then, a mixed solution of 0.4 mol of triethylamine and 100 g of methylene chloride was dropwise added, and the resultant mixture was further stirred for 30 minutes to complete the reaction. A hydrochloride of triethylamine was separated by filtration. The thus-obtained filtrate was washed with 1,000 ml of 0.1 N hydrochloric acid, and then washing with 1,000 ml of water was repeated four times. After drying with sodium sulfate, evaporation was carried out at 75° C., to obtain a crystal of a yellow solid. Th...
example b1-1
Production of Cured Product
[0059] The cyanate ester G65C of biphenyl novolak obtained in Example 1 in an amount shown in Table 1 was added in a short-neck flask. The cyanate ester G65C was molten under heat at 150° C. and degassed with a vacuum pump. Then, zinc octylate was added and the resultant mixture was stirred for 1 minute. The stirred mixture was casted in a mold composed of a glass plate (120 mm×120 mm×5 mmt), a polyimide film (Kapton 200H: DU PONT-TORAY CO., LTD.) and an O-ring made of fluoro rubber (S-100: MORISEI Co., Ltd.), and it was cured by heating in an oven at 170° C. for 1 hour and at 230° C. for 9 hours. After cooling, the polyimide film was removed by polishing to obtain a cured product of cyanate ester compound.
[0060] The obtained cured product was evaluated for properties by the following methods.
[0061] Glass transition temperature (Tg): Obtained according to a dynamic viscoelasticity measurement (DMA). The measurement was carried out at an oscillation freq...
example b1-2
[0065] A cured product was obtained in the same manner as in Example B1-1 except that 50 parts by weight of the G65C obtained in Example 1 and 50 parts by weight of bisphenol A dicyanate Skylex, supplied by Mitsubishi Gas Chemical Company, Inc., were used.
[0066] Table 1 shows the evaluation results of physical properties of the cured product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com