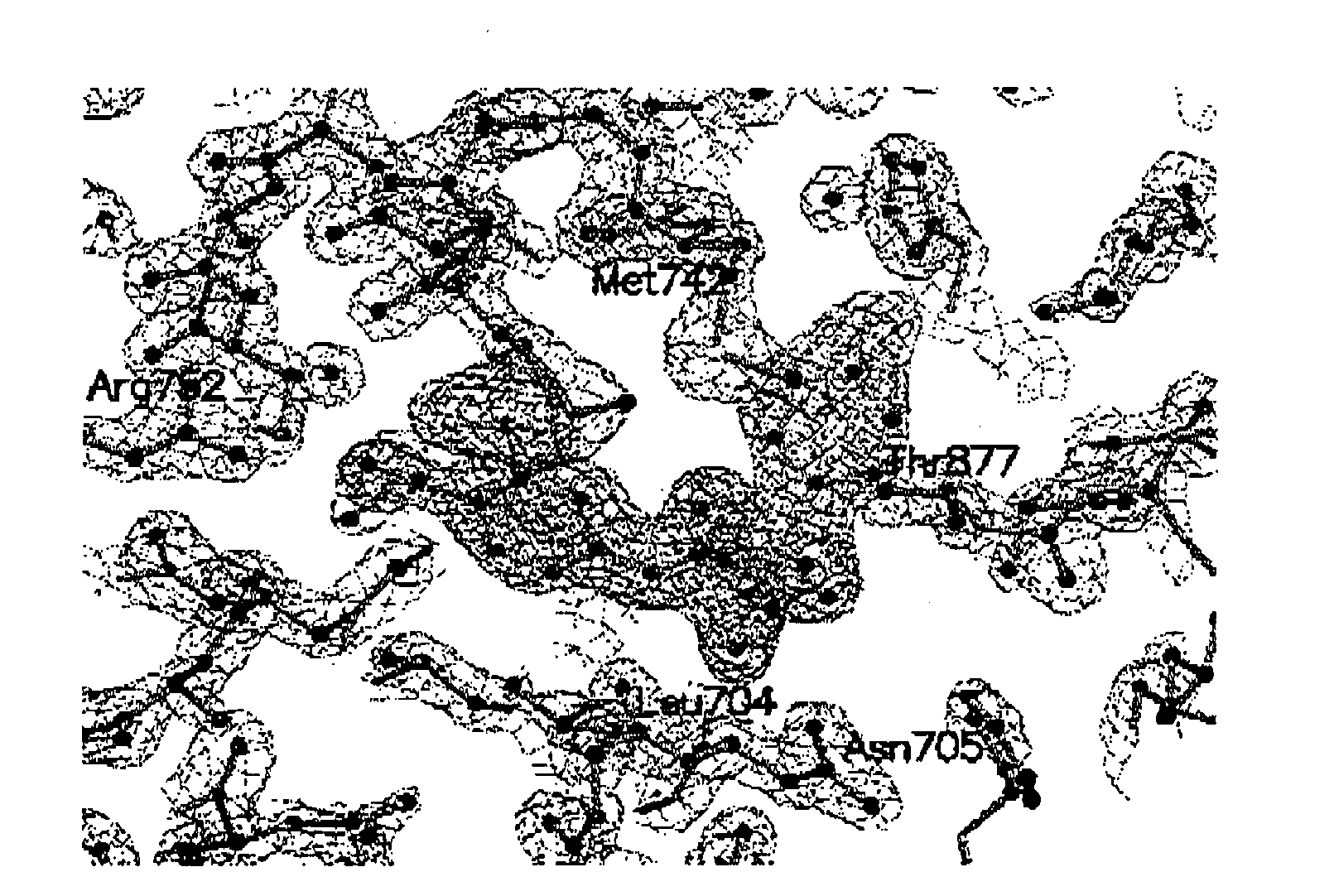
Selective androgen receptor modulators, analogs and derivatives thereof and uses thereof
a selective androgen receptor and modulator technology, applied in the field of nuclear hormone receptor binding compounds, can solve the problems of reducing sexual sensitivity, affecting the onset of osteoporosis in the elderly, and difficult subject of contraception, so as to reduce the incidence, delay the onset, and reduce the severity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
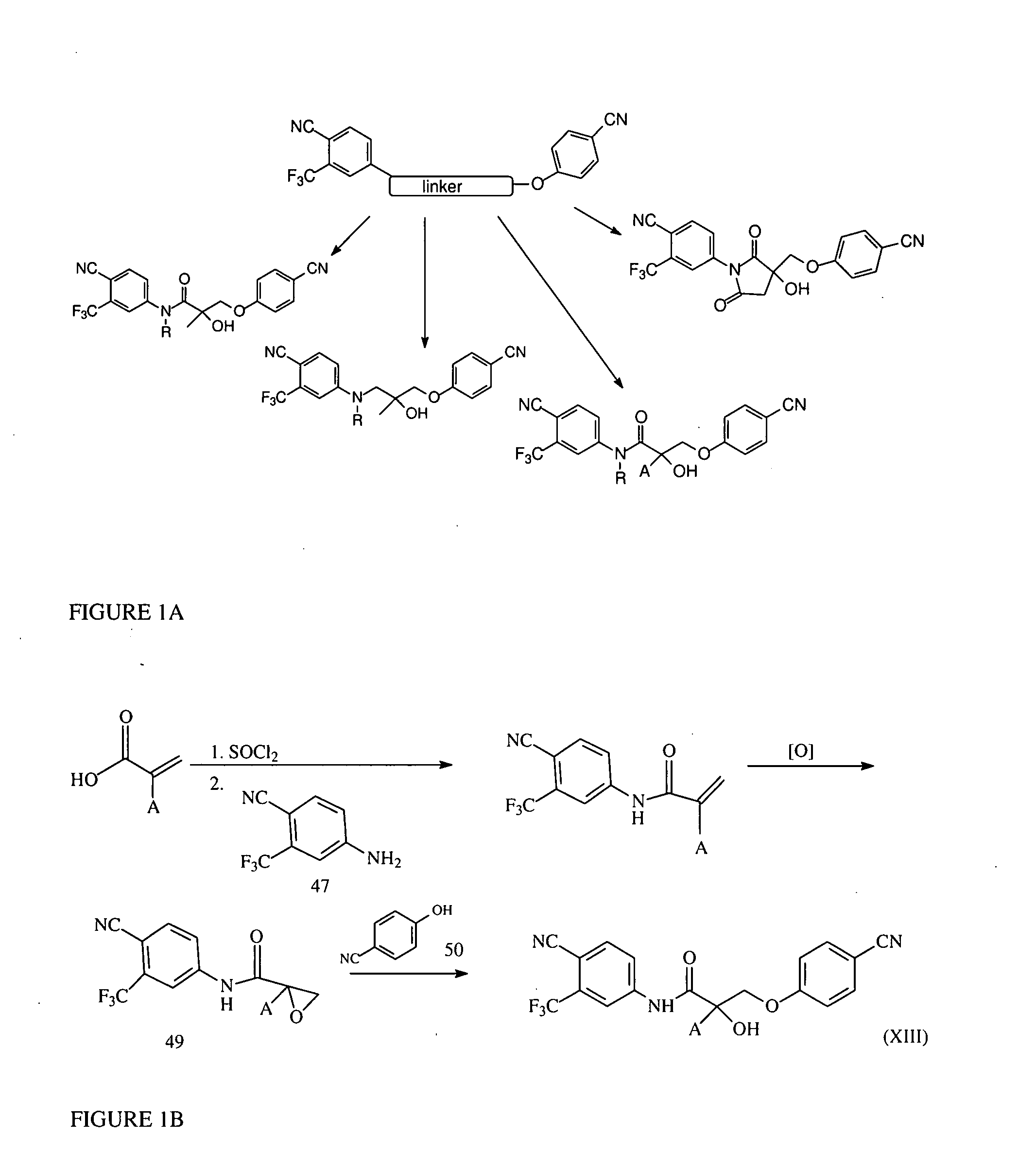
Synthesis of Compound XIII
[1010]2-substituted acrylic acid (compound 46) is reacted with 4-cyano-3-trifluoromethyl-aniline followed by epoxidation. An opening of the epoxide ring with p-CN-phenol in the presence of potassium carbonate yields compound XIII, as presented in FIG. 1B.
example 2
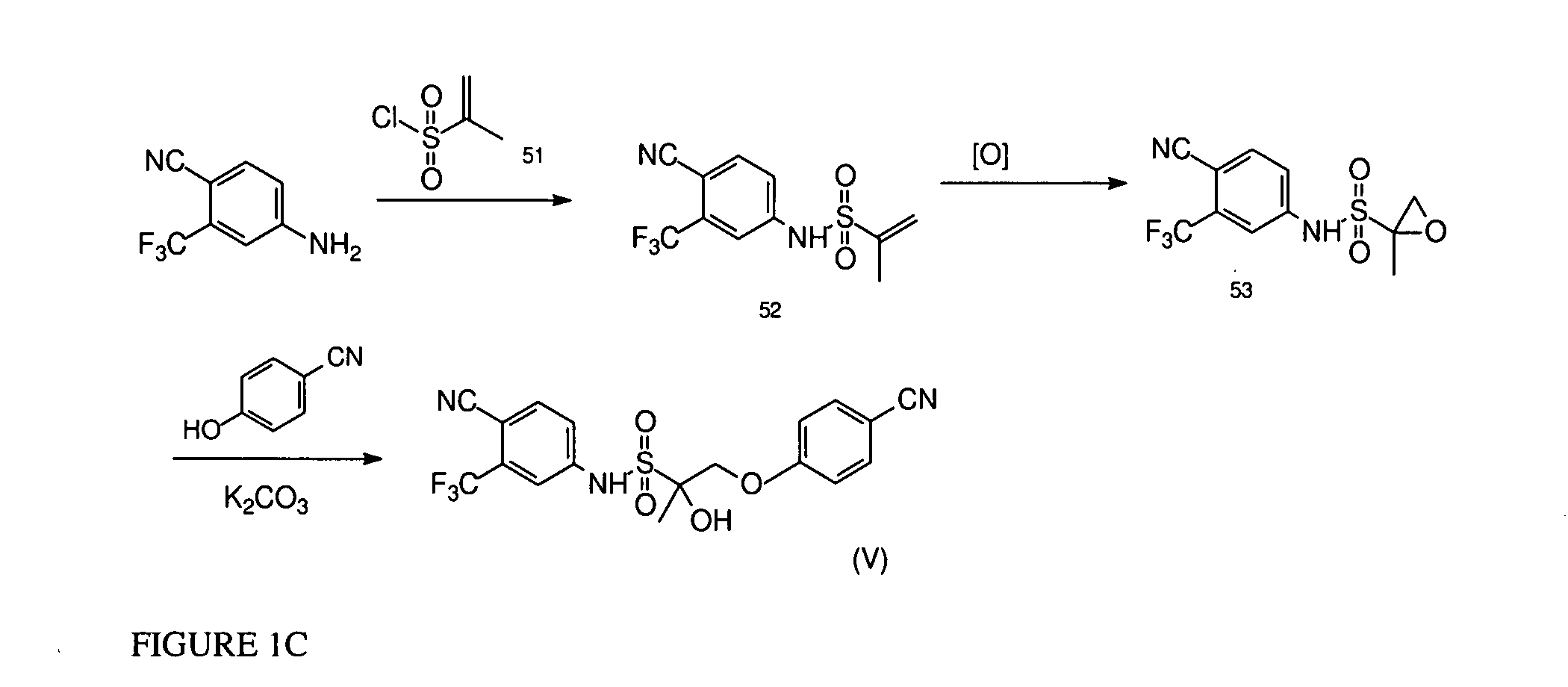
Synthesis of Compound V
[1011]4-cyano-3-trifluoromethyl-aniline is reacted with propene-2-sulfonyl chloride (51), and subjected to epoxidation followed by opening of the epoxide ring with p-CN-phenol in the presence of potassium carbonate to yield compound V, as presented in FIG. 1C.
example 3
Synthesis of Compound XI
[1012]Oxiranylmethyl-carbamic acid tert-butyl ester (54) was opened with p-CN-phenol to yield compound 55, followed by deprotection with TFA to yield amino alcohol 56. Compound 56 was introduced in an electrophilic substitution reaction with 4-fluoro-2-trifluoromethyl-benzonitrile (57) to form compound 58. Oxidation of 58 to corresponding carbonyl derivative 59 which was used in further addition reactions to yield compound XI which as presented in FIG. 1D.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com