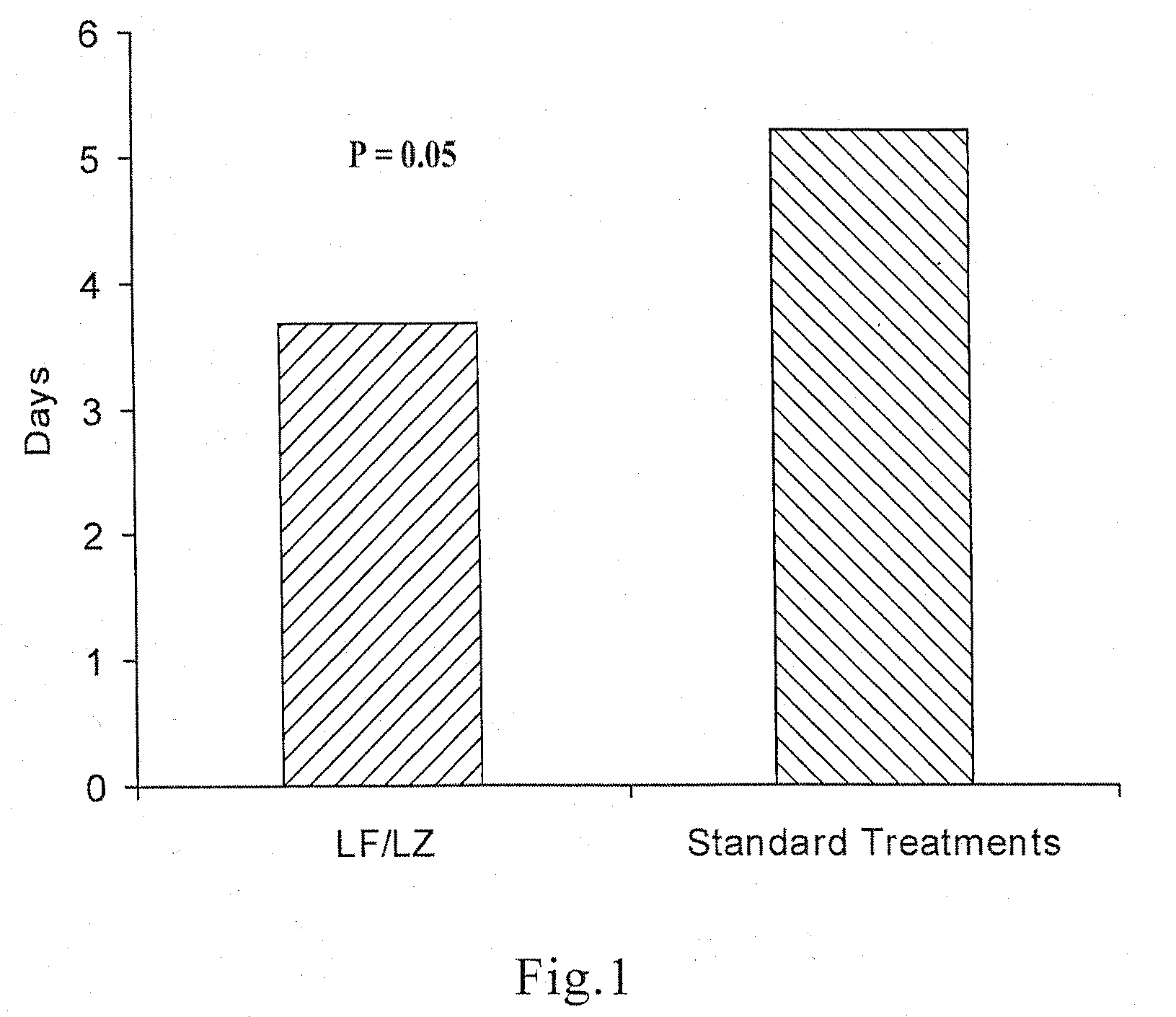
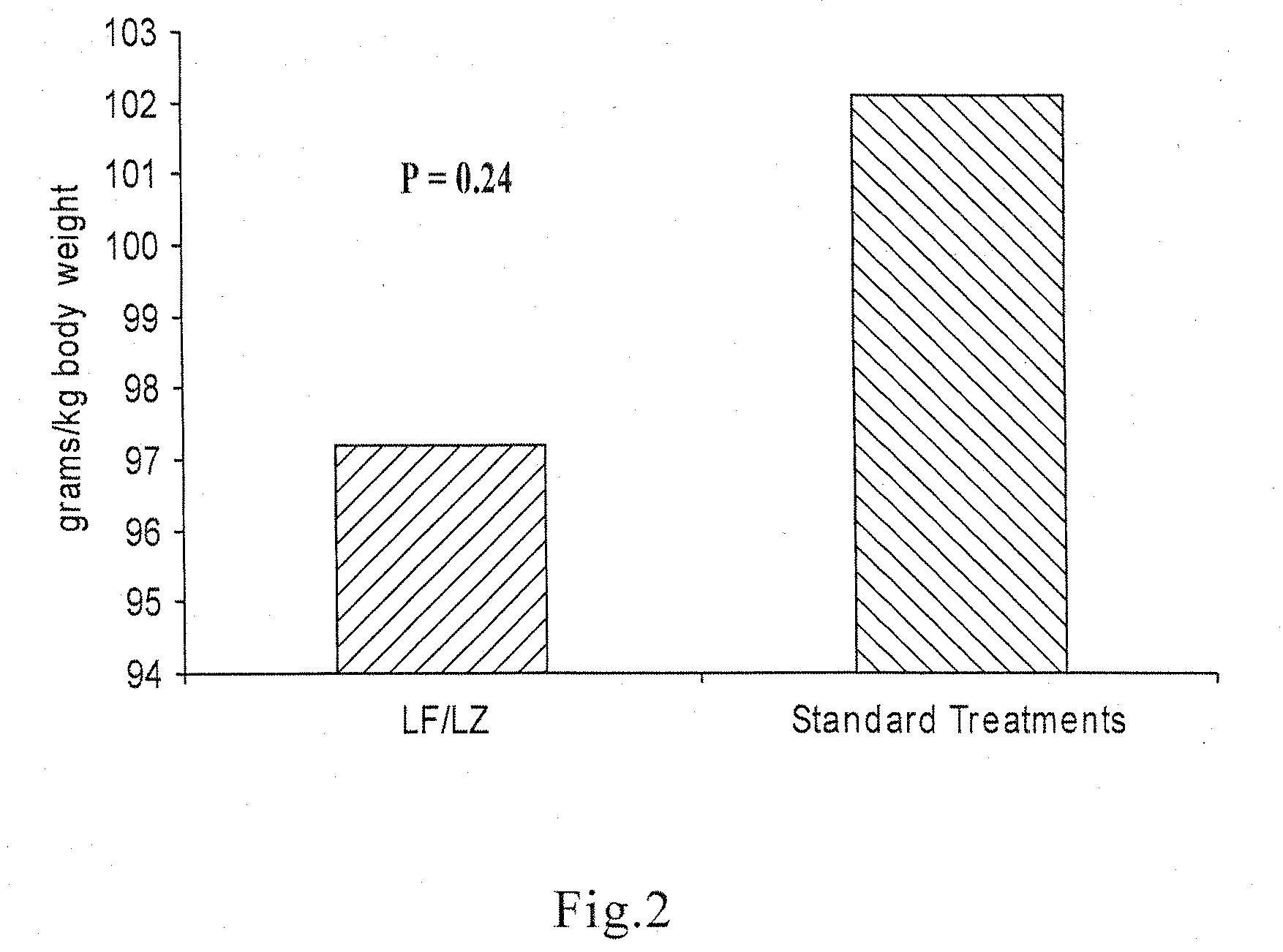
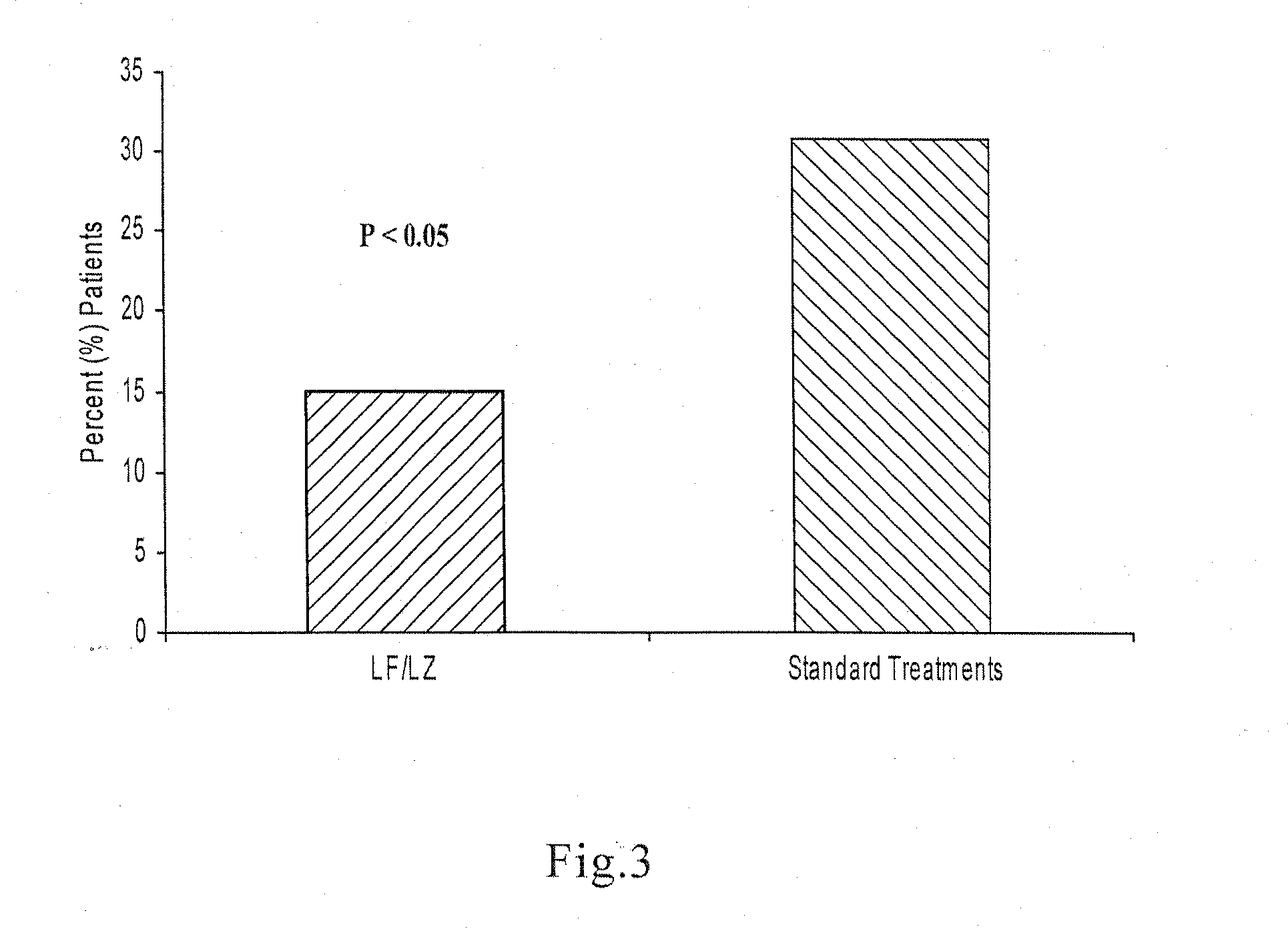
Oral formulations for enteric disorders and/or rehydration
a technology for enteric disorders and oral formulations, applied in the field of oral formulations for enteric disorders and/or rehydration, can solve the problems of increasing the cost of rice-based materials, not being superior for children with acute non-cholera diarrhea, and cholera patients' diarrhea duration and other problems, to achieve the effect of enhancing the recovery rate, reducing the duration and/or severity of intestinal diseases, and improving rehydration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Expression of Recombinant Human Lactoferrin
A. An Expression Vector for Human Lactoferrin Expression in Transgenic Rice
[0094]The complete nucleotide sequence of human mammary gland lactoferrin was codon optimized and synthesized by Operon Technologies (CA, USA). Human milk lactoferrin gene (Genbank accession number: HSU07642) was re-synthesized with codons most frequently used in translation of rice seed proteins in order to obtain optimal level of expression. Although the numbers of codons changed accounted for 22.46% of the entire sequence, the amino acid composition remained identical to non-recombinant human lactoferrin. The plasmid containing the codon-optimized gene was called Lac-ger. Lac-ger was digested with SmaI / XhoI and the fragment containing the lactoferrin gene was cloned into pAPI141 that was partially digested with NaeI and completely digested with XhoI. For expression of hLF in rice seeds, the codon-optimized gene was operably linked to the rice endosperm-specific gl...
example 2
Purification of Recombinant Human Lactoferrin
[0097]To prepare an oral rehydration solution supplemented with recombinant human lactoferrin, recombinant human lactoferrin was purified from rice flour. A transgenic rice line (164-12) expressing high levels of rhLF was selected. This line, now named as LF164, was planted two generations per year, alternating field planting in summer and greenhouse planting in winter. For protein purification, paddy rice expressing rhLF was de-hulled by using a de-huller (Rice Mill, PS-160, Rimac, Fla.), and then ground to flour (average particle size of 100 mesh) using a hammer mill (8WA, Schutte—Buffalo, N.Y.).
[0098]Protein extraction from transgenic flour was performed by mixing two kg of rice flour and 20 L of extraction buffer (0.02 M sodium phosphate pH 6.5 and 0.3 M sodium chloride) in a 50 L tank for 1 h. At the end of the mixing period, the suspension was allowed to settle overnight or centrifuged at 3750 rpm. In both cases, the supernatant was...
example 3
Production and Purification of Recombinant Human Lysozyme
[0102]Recombinant human lysozyme is produced in the LZ159 rice variety derived from Oryza sativa, Japonica, Taipei 309. The rice is dehusked and milled to an average of 100 mesh flour using standard food industry procedures. Recombinant human lysozyme is extracted from ground rice flour using 0.02 M acetate buffer with 0.3 M NaCl pH 4.5. After 1.0 to 2.0 hours of extraction, the solid rice flour is separated from the liquid phase by centrifugation. The liquid phase, containing the soluble protein, is filtered and concentrated. At this point the product is a protein concentrate that is >10% lysozyme protein. The concentrate is further purified to an isolate using ion exchange chromatography. The concentrate is loaded on a column of SP Sepharose Big Bead Media, the column is washed and the bound lysozyme eluted with 0.8 M sodium chloride. The isolate is ultrafiltered to remove excess salt and concentrated prior to lyophilization...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com