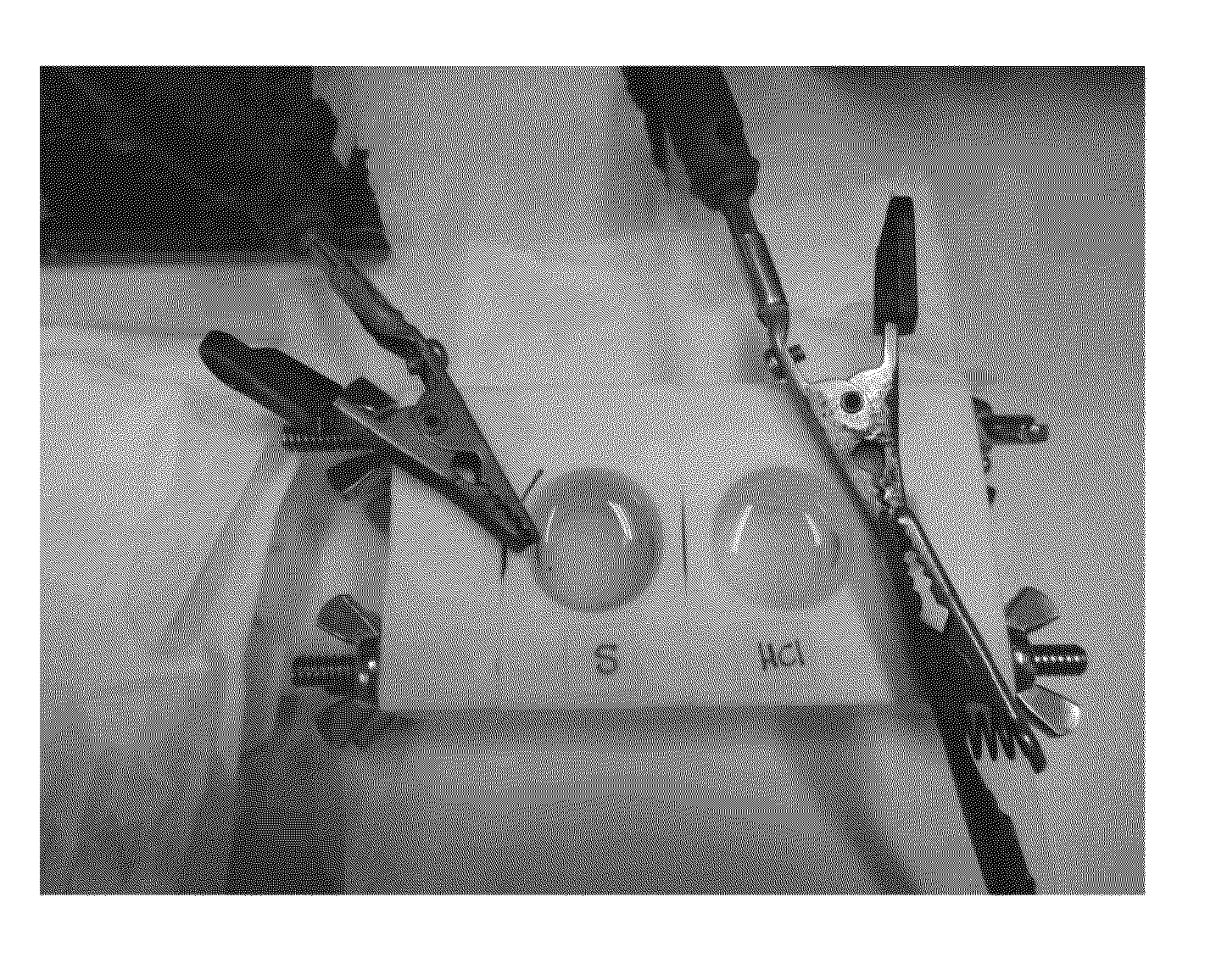Method and electrochemical device for low environmental impact lithium recovery from aqueous solutions
a technology of electrochemical devices and lithium, which is applied in the direction of electrochemical machining apparatus, diaphragms, fuel and secondary cells, etc., can solve the problems of large volume of chemical waste, profound alteration of water balance, and fragile ecosystem, and achieve low environmental impact and high selective effect of lithium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
LiMnO4 Preparation
[0107]LiMn2O4 was synthesized using solid state chemistry. 0.377 g of Li2CO3 (Aldrich) and 1.74 g of MnO2 (Aldrich) were thoroughly mixed at a molar 0.51:2 ratio in a mortar, and heated at 350° C. for 12 hours. Samples were subsequently heated at 800° C. for 24 hours with 3 cycles of grinding and firing. The resulting powder was characterized by scanning electron microscopy (SEM) and X-ray Diffraction (XRD).
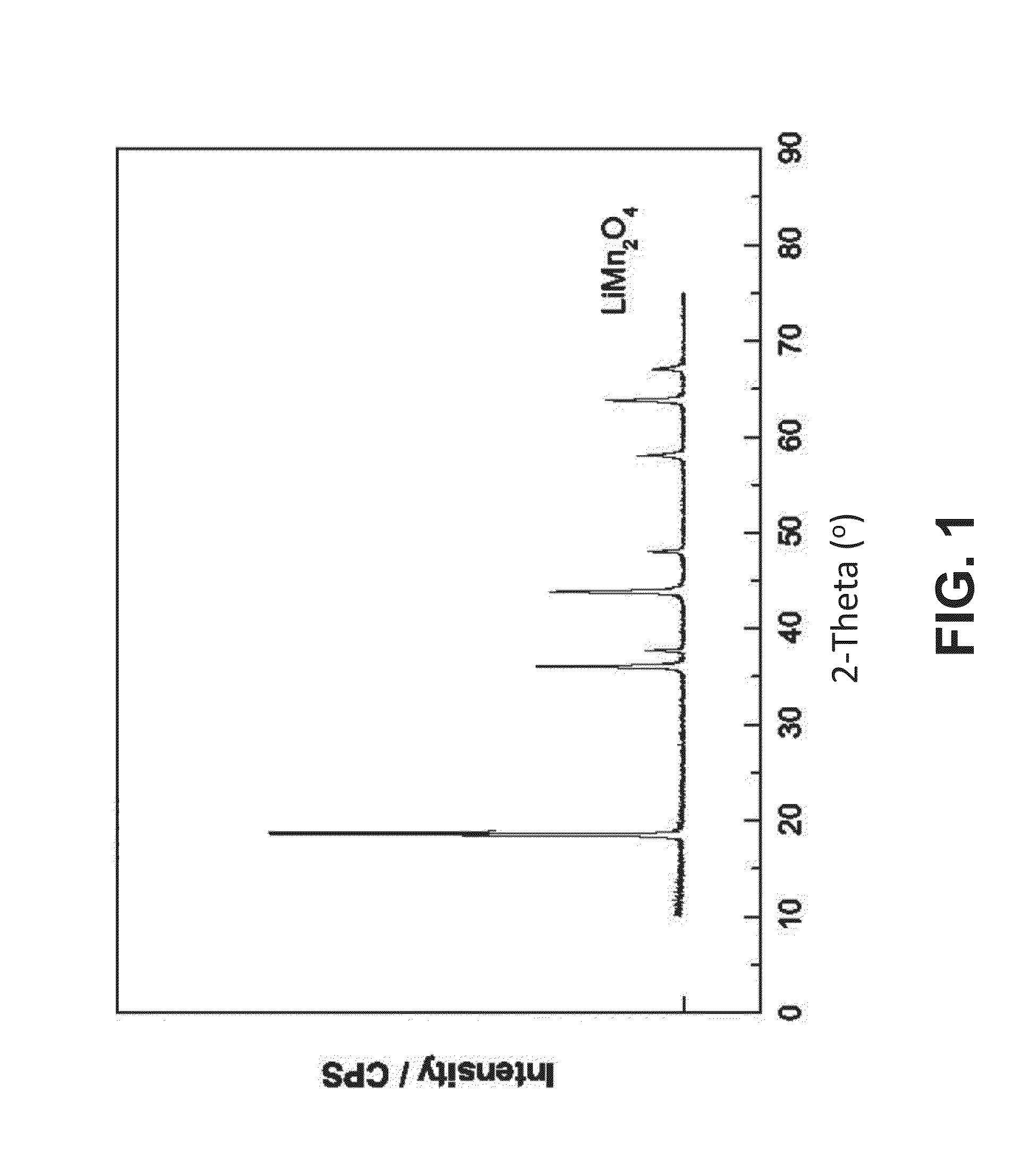
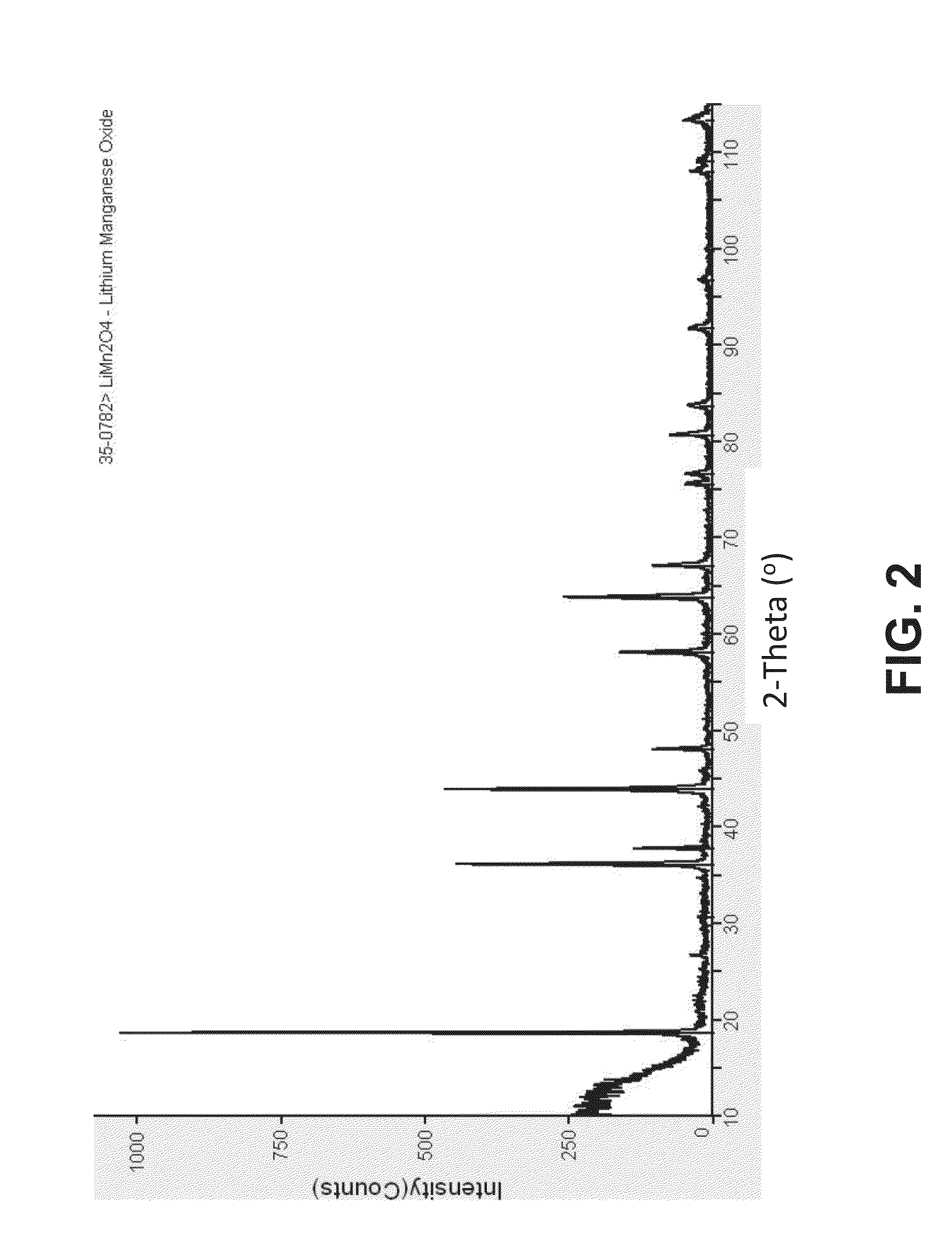
[0108]An X-ray diffractogram of a LiMn2O4 sample obtained according to the method described above is shown in FIG. 2. The comparison of such diffractogram with an X-ray diffractogram of a LiMn2O4 standard (FIG. 1) indicated that a single phase mixed oxide was obtained. SEM examination of the resulting LiMn2O4 samples showed very well formed crystals with average size of several nanometers to a micrometer (see FIG. 3).
example 2
Preparation of Carbon Felt embedded with LiMn2O4
[0109]Conductive carbon felt electrodes (National Electric Carbon Products, a division of Morgan Specialty Graphite; Greenville, S.C., US) were cut into 20×10×3 mm pieces, thoroughly washed with 1:1 isopropanol:Milli-Q water and finally rinsed with Milli-Q water (FIG. 4).
[0110]The synthesized LiMn2O4 powder was loaded onto the carbon felt electrode as a slurry prepared with 80% Li—Mn oxide, 10% carbon black (Chevron Phillips SHAWINIGAN BLACK®) and 10% PVC (polyvinyl chloride) in dichloromethane. Subsequently, the carbon felts were dried at 60° C. under vacuum during 24 hours.
[0111]The LiMn2O4 loaded carbon felt electrodes were subsequently subjected to electrolysis to de-lithiate the oxide while keeping the highly selective crystal structure to allow the intercalation of lithium ions. The oxide loaded carbon electrode was placed in one of the compartments in a two compartment TEFLON® cell, and a platinum counterelectrode was placed in...
example 3
Preparation of Silver Chloride Reversible Electrodes
[0114]Several approaches were used to prepare chloride reversible electrodes.
[0115]In one chloride reversible electrode preparation, silver was directly deposited from a commercial silver cyanide bath (Argex, Laring S. A., Argentina) by holding the potential of the carbon felt at −0.1 V. The silver cyanide bath had been previously sonicated in isopropanol for 30 minutes and rinsed with Milli-Q water. Silver crystals of 100 nanometers to 1 micrometer were obtained on the conductive carbon fibers.
[0116]In another chloride reversible electrode preparation, a layer-by-layer polyelectrolyte multilayer was deposited on the carbon fibers as described, for example, in Rubner et al., Langmuir 18:3370-75 (2002), and Vago et al., Chem. Commun. 5746-48 (2008). The polyelectrolyte multilayer functioned as a nanoreactor to confine the silver ions. The silver ions were further reduced chemically with 5 mM sodium borohydride or reduced electro-che...
PUM
| Property | Measurement | Unit |
|---|---|---|
| altitude | aaaaa | aaaaa |
| DC voltage | aaaaa | aaaaa |
| DC voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com