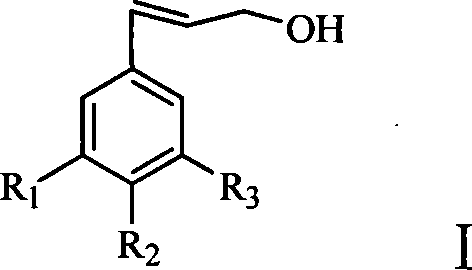
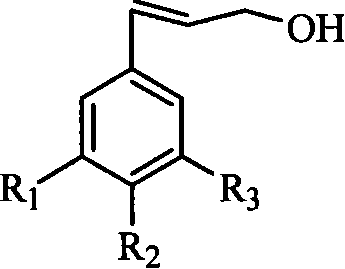
Substituted benzyl ethylene derivant and method of preparing the same and use thereof
A derivative, the technology of phenylpropylene, applied in the field of preparation of phenylpropylene derivatives, can solve the problems of limiting the universal applicability of drugs, malignant killing of normal cells, and unsatisfactory, and achieves feasible market prospects, low cost, The effect of the simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
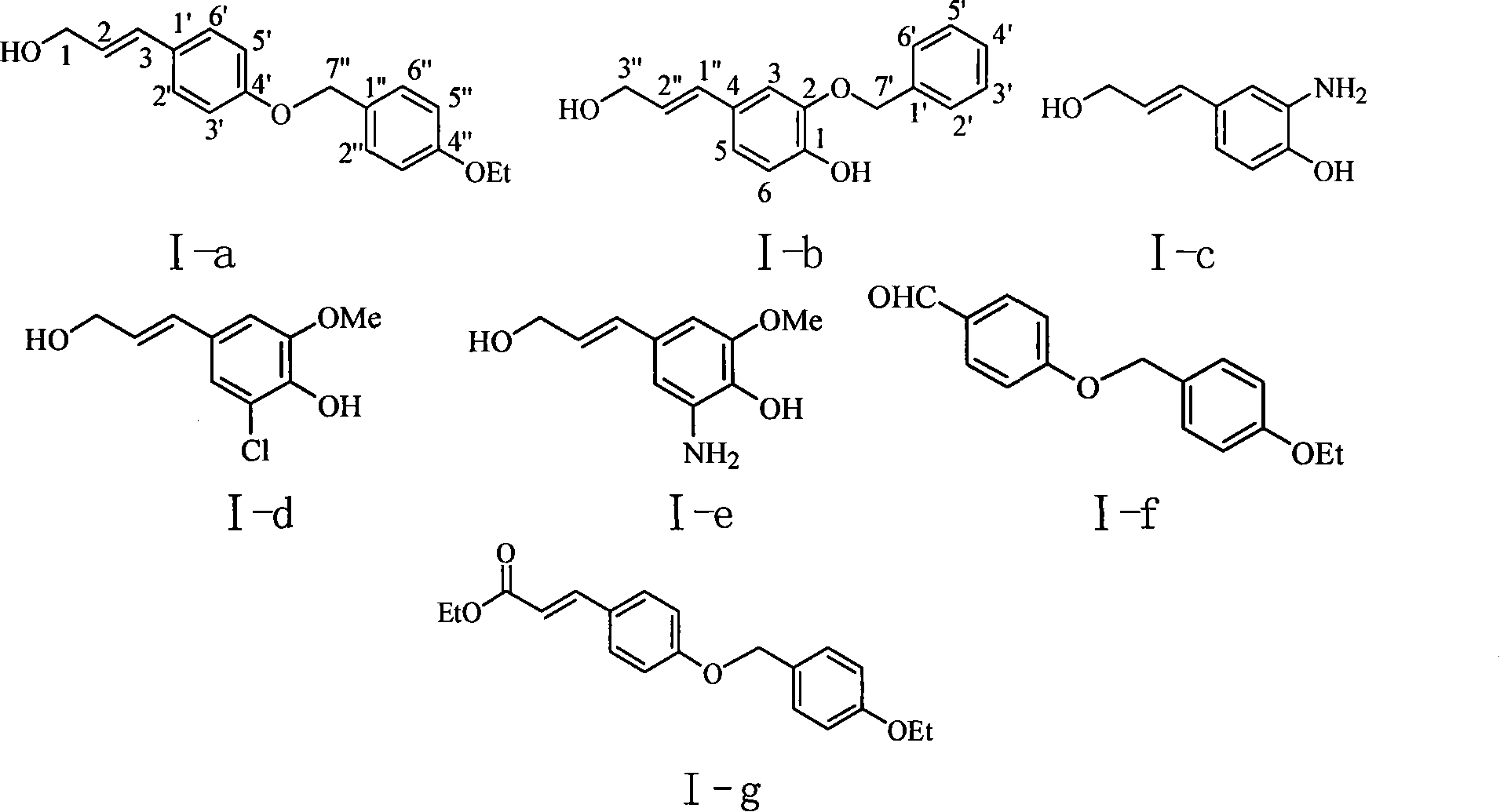
[0032] Example 1 : Preparation of important intermediate I-f (4-(4-ethoxybenzyloxy)-benzaldehyde)
[0033]
[0034] This example relates to the general synthesis method of substituted benzaldehyde series compounds which are key intermediates of substituted phenylpropene derivatives with cytotoxic activity. It specifically relates to the synthesis of compound I-f (4-(4-ethoxybenzyloxy)-benzaldehyde). Dissolve syringaldehyde (298 mg, 1.6 mmol) in 20 ml of acetone, add anhydrous potassium carbonate (567 mg, 4.1 mmol), stir for ten minutes, and then add p-ethoxybenzyl bromide (537 mg, 2.5 mmol) 10 ml of acetone solution, reflux for 4 hours. Thin-layer chromatography (TLC) analysis showed that the reaction of the raw material was basically complete, cooled to room temperature, filtered to remove potassium carbonate, and distilled off the solvent acetone. The crude product obtained after concentration was separated by column chromatography to obtain 281 mg of white solid with ...
Embodiment 2
[0036] Example 2 : the preparation of important intermediate I-g (3-[4-(4-ethoxybenzyloxy group) phenyl]-2-propenoic acid ethyl ester (2E))
[0037]
[0038]This example involves the general synthesis of substituted phenylacrylates with cytotoxic activity. It specifically relates to the synthesis of compound I-g (3-[4-(4-ethoxybenzyloxy)phenyl]-2-propenoic acid ethyl ester (2E)). Compound I-f (256 mg, 1.0 mmol) was dissolved with pyridine (5 mL) in a 100 mL three-necked flask, added dropwise into 0.2 mL of piperidine, and monoethyl malonate (198 mg, 1.5 mmol) was added, Reflux for 2 hours. Cool to room temperature, add 2M hydrochloric acid to neutralize pyridine, partition and extract with water and ethyl acetate, concentrate the organic layer, and purify by column chromatography (petroleum ether / ethyl acetate=6:1, crude product / silica gel=1:30) to obtain a white solid , the yield was 75.1%.
[0039] Compound I-g: white solid, melting point: 103-104°C, Rf (n-hexane / eth...
Embodiment 3
[0040] Example 3 : the preparation of compound I-a (3-[4-(4-ethoxybenzyloxy) phenyl]-2-propene-1-alcohol (2E))
[0041]
[0042] Lithium aluminum hydride (387 mg, 10.2 mmol) and aluminum trichloride (427 mg, 32.0 mmol) were placed in a three-necked flask, 10 ml of tetrahydrofuran was added under the protection of an inert gas, cooled to 0 ° C in an ice-salt bath, and added by implementing Compound I-g (3-[4-(4-ethoxybenzyloxy)phenyl]-2-propenoic acid ethyl ester (2E)) obtained in Example 2 was 1.043 g (3. mmol), and stirred for 1.5 hours. Add water to decompose excess lithium aluminum hydride, then adjust the pH to acidic with 1M hydrochloric acid, extract with ethyl acetate, wash the organic phase with saturated brine, dry overnight with anhydrous sodium sulfate, filter, concentrate the filtrate to obtain a crude product, and purify by column chromatography ( Petroleum ether / ethyl acetate=4:1, crude product / silica gel=1:50) to get 3-[4-(4-ethoxybenzyloxy)phenyl]-2-propen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com