Catalytic esterification method for sulfonic group functionalization morpholine hyamine ion liquid
A quaternary ammonium salt ion, functionalized technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., to achieve high yield, good thermal stability, conversion full effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
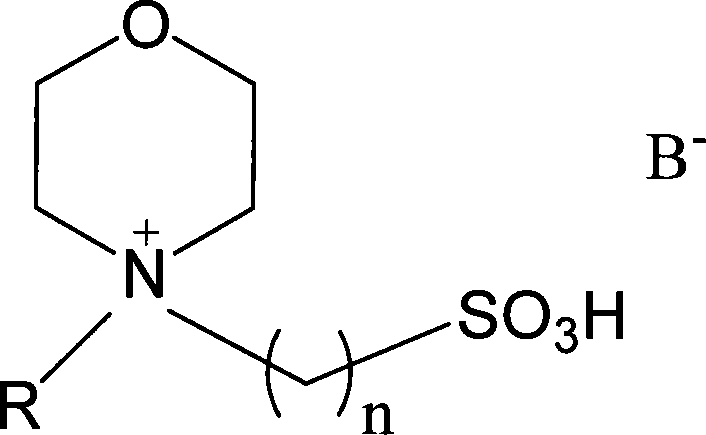
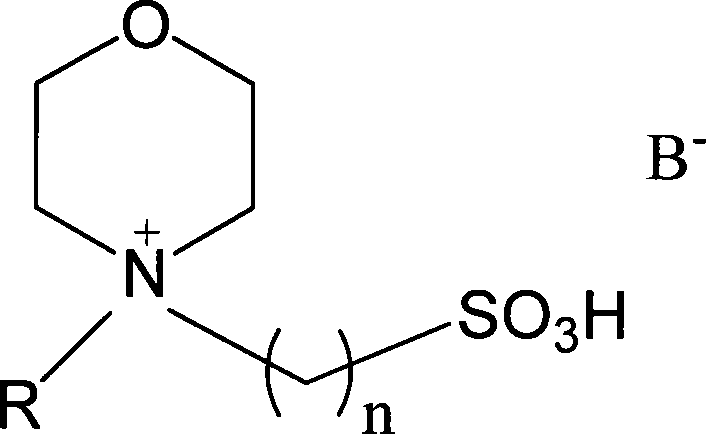
[0027] Embodiment 1: Take by weighing 0.05mol of acetic acid, 0.05mol of methanol, 0.01mol of ionic liquid N-(3-sulfonic acid group) propylmorpholine bisulfate; add ionic liquid, methyl alcohol, acetic acid successively with stirrer, In a round bottom flask with a thermometer and a reflux condenser, stir magnetically, and heat and reflux at 40°C for 3 hours; let stand for stratification, and take the upper esterification product, with a yield of 50.69% and a selectivity of 100%; Repeated use after vacuum dehydration.
Embodiment 2
[0028] Embodiment 2: Take by weighing 0.05mol of acetic acid, 0.065mol of ethanol, and 0.01mol of ionic liquid N-(3-sulfonic acid group) propyl morpholine bisulfate; ionic liquid, ethanol, acetic acid are added successively with agitator, In a round bottom flask with a thermometer and a reflux condenser, stir magnetically, and heat and reflux at 80°C for 3 hours; let stand to separate layers, and take the upper esterification product, the yield is 93.4%, and the selectivity is 100%; Repeated use after vacuum dehydration.
Embodiment 3
[0029] Embodiment 3: Take by weighing 0.05mol of acetic acid, 0.065mol of propanol, 0.01mol of ionic liquid N-(3-sulfonic acid group) propyl morpholine bisulfate; add ionic liquid, propanol, acetic acid successively with stirring In a round-bottomed flask with a device, a thermometer, and a reflux condenser, magnetically stir, and heat and reflux at 80° C. for 3 hours; let stand to separate layers, and pipette the upper esterification product. The yield is 83.7%, and the selectivity is 100%. The liquid is vacuum dehydrated and reused.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com