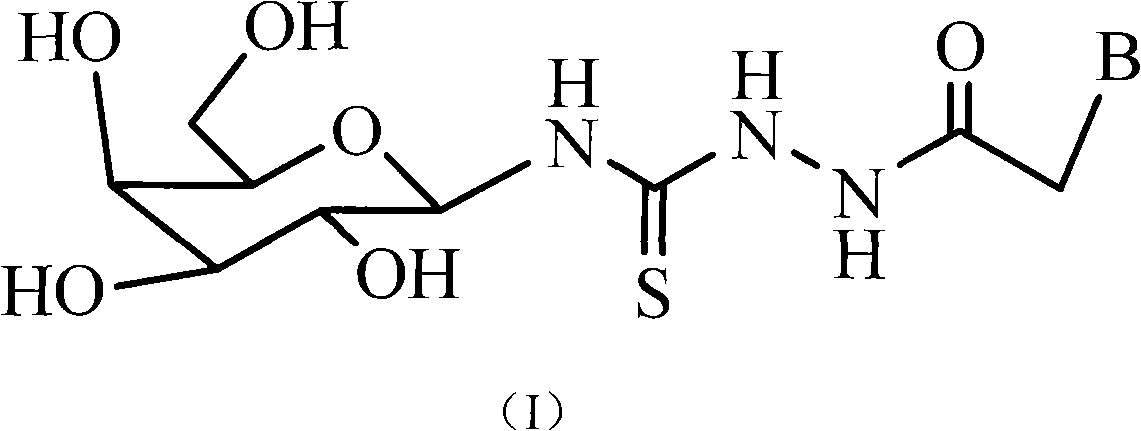
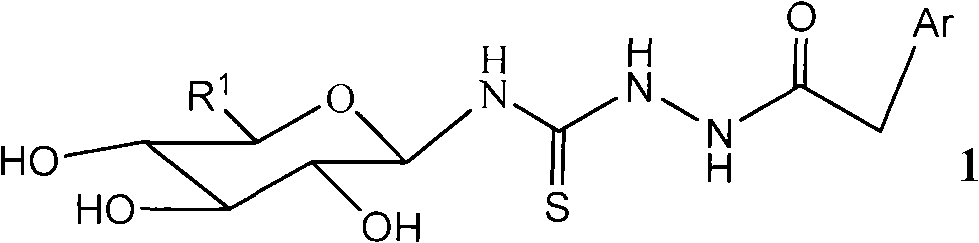
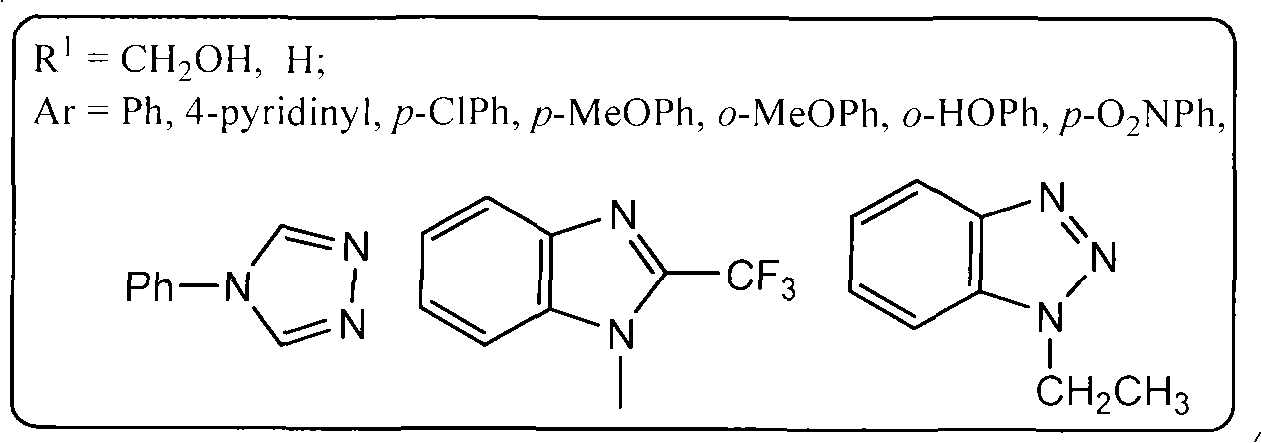
Galactosyl thiourea heterocyclic compounds, synthetic method thereof and antineoplastic applications
A technology of heterocyclic compounds, galactose, applied in the field of galactosylthiourea heterocyclic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 : Synthesis of 1-adeninyl-acetyl-4-(1'-N-β-D-galactosyl)-thiosemicarbazide (compound number is a)
[0053] (1) Synthesis of 1-bromo-2,3,4,6-tetra-O-acetyl-α-D-galactose
[0054]Add red phosphorus (4.5g, 3.63mmol) and 60mL glacial acetic acid into a 250mL three-necked flask, add bromine water (12mL, 234.10mmol) with a dropping funnel while stirring, and stir the mixed reaction solution at room temperature for half an hour, filter Discard red phosphorus. Add fully acetylated D-galactose (39g, 100mmol) and react at the same temperature until all the fully acetylated D-galactose of the reactant as detected by thin layer chromatography disappears. The glacial acetic acid was removed under reduced pressure, the residue was extracted three times with saturated sodium carbonate and chloroform, the organic extract was washed three times with saturated brine, dried over anhydrous sodium sulfate, and the organic solvent was removed under reduced pressure to obtain 1-br...
Embodiment 2
[0065] Example 2 : Synthesis of 1-benzimidazolyl-acetyl-4-(1'-N-β-D-galactosyl)-thiosemicarbazide (compound number b)
[0066] (1) Synthesis of 1-bromo-2,3,4,6-tetra-O-acetyl-α-D-galactose
[0067] The method and conditions of (1) in Example 1 are adopted for synthesis.
[0068] (2) Synthesis of 2,3,4,6-tetra-O-acetyl-α-D-galactosyl isothiocyanate
[0069] Synthesize using the method and conditions of (2) in Example 1.
[0070] (3) Synthesis of ethyl benzimidazolyl acetate
[0071] The method and conditions of (3) in Example 1 were used for synthesis, only adenine was changed to benzimidazole.
[0072] (4) Synthesis of benzimidazolyl acetylhydrazide
[0073] The method and conditions of (4) in Example 1 were used for synthesis, only ethyl adenyl acetate was changed to ethyl benzimidazolyl acetate.
[0074] (5) 1-Benzimidazolyl-acetyl-4-(1'-N-2',3',4',6'-tetra-O-acetyl-β-D-galactosyl)-amino Synthesis of Thiourea
[0075] Synthesize using the method and conditions of (5...
Embodiment 3
[0079] Example 3 : Determination of inhibitory activity against human lung cancer cell line (PG)
[0080] Using the MTT method, human lung cancer cell line (PG) was used as the target cell, and the cell culture medium was 1640 medium containing 10% BCS. After the logarithmic growth phase cells were digested with trypsin, the cell density was adjusted to 3-5×10 3 per well, seeded in a 96-well culture plate, 100 μl per well, placed in a temperature of 37 ° C, CO 2 Incubate for 24 hours in an incubator with a content of 0.5%. Add 50 μl / well of the prepared samples with different concentrations to the 96-well culture plate. The positive control drug Zidovudine (AZT) with different dilution concentrations is used as the positive control, and the temperature is 37 ° C, CO 2 Continue culturing for 48 hours in an incubator with a content of 0.5%. Take out the 96-well culture plate, add 20 μl of 5 mg / mL MTT to each well, and continue culturing for 4 hours. Take out the culture plate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com