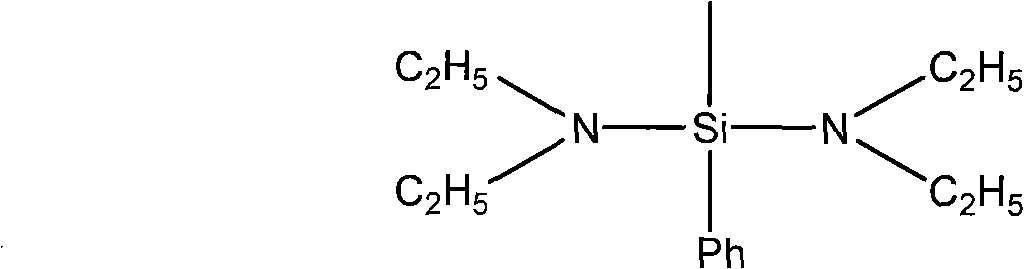
Preparation of bis(N,N-diethyl) aminomethyl phenyl silane
A technology of diethylaminomethylphenylchlorosilane and aminomethylphenylsilane, which is applied in the field of organic silicon preparation and can solve the problems of inability to obtain high-purity methylphenyldichlorosilane and difficulty in separation and purification , to achieve the effect of convenient process, easy access to raw materials and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
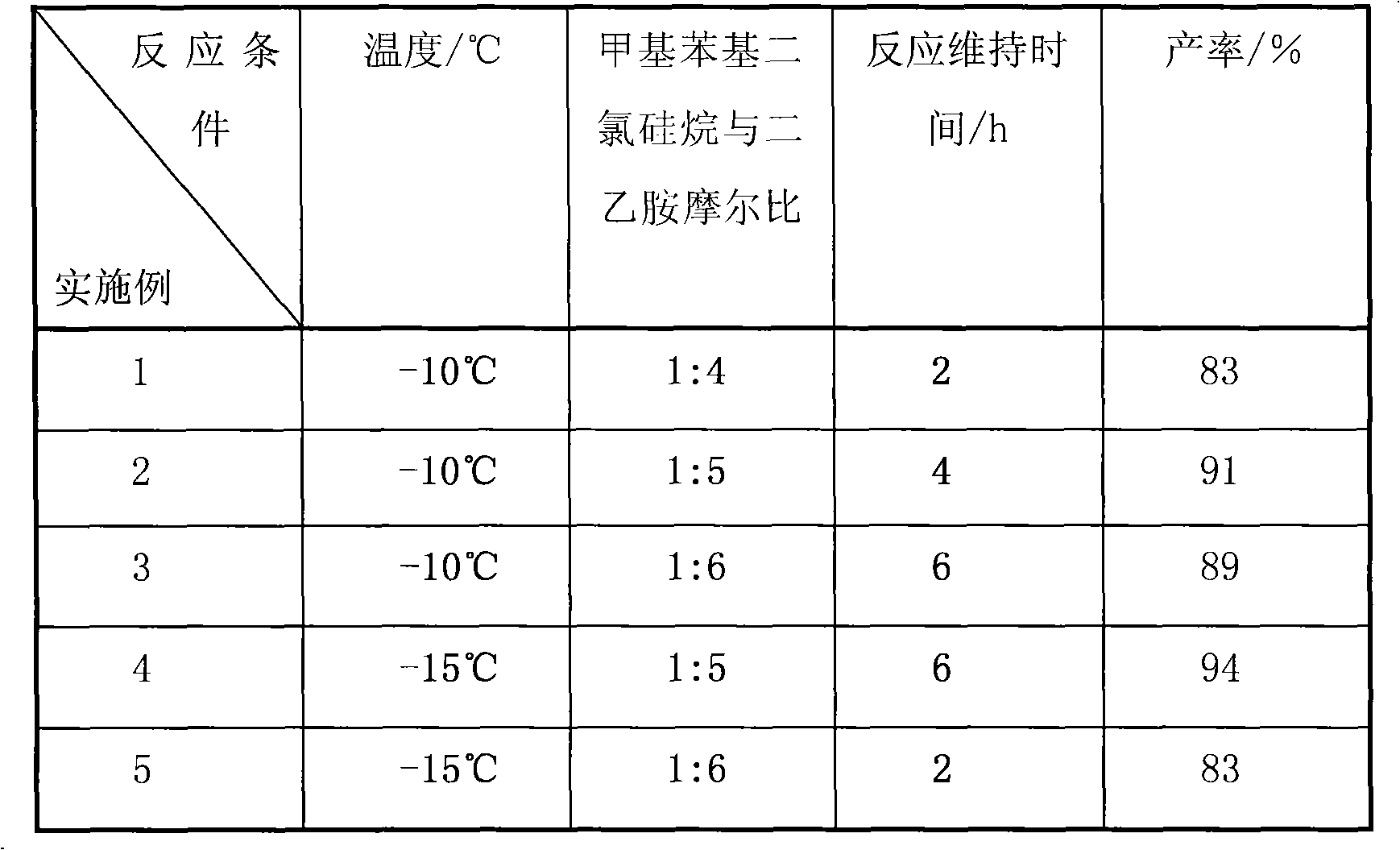
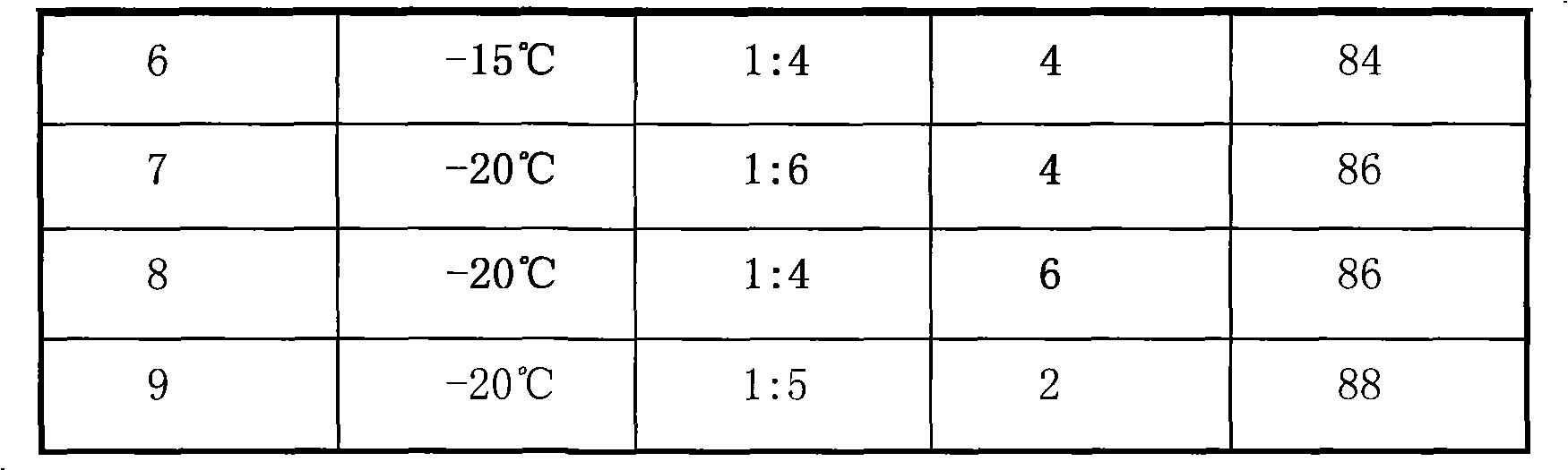
Embodiment 1~9
[0028]Under the protection of nitrogen, add 150mL of dichloromethane and a certain amount of diethylamine that were treated with calcium hydride drying and reflux into a 500mL four-port container equipped with an electric stirrer, a constant pressure dropping funnel and an air port device after drying. In the round-bottomed flask, when the temperature of the reaction system reaches -20°C, slowly add 19.1 g (0.1 mol) of methylphenyldichlorosilane with a purity of 97wt% dropwise for 2 hours. After the dropwise addition, the temperature was maintained for 2h, and then the reaction was stopped. The reaction mixture was transferred to a rotary evaporator, and 80 mL of dichloromethane was recovered, then the concentrated reaction mixture was lowered to room temperature, airtight, protected from light and allowed to stand for 24 hours, and then filtered by centrifugal filtration to remove ammonium salts, and the filtrate was GC -MS to analyze and calculate the yield of bis(N,N-diethy...
Embodiment 10
[0033] After mixing the 233g filtrate obtained in Examples 1 to 9, add it to a 500mL three-necked flask, distill under reduced pressure, collect 199.4°C / 2.0Kpa cuts, and obtain 156g of a light yellow solution, which consists of 67.92wt% (N,N-di Ethyl) aminomethylphenyl chlorosilane, 22.12wt% of two (N, N-diethyl) aminomethylphenylsilane, 6.24wt% unreacted methylphenyldichlorosilane, 0.96wt % of bis(N,N-diethyl)aminophenylchlorosilane and 2.75 wt% of methylphenylcyclotrisiloxane.
[0034] Add 53.3g (0.73mol) of diethylamine that adopts the method described in Example 1 to dry handle in a 500mL four-neck round bottom flask equipped with an electric stirrer, a constant pressure dropping funnel, and an air inlet device, and then add 100mL of the dried dichloromethane solution, when the temperature of the reaction mixture reaches -15°C, slowly add 100g of the fractions collected by the above vacuum distillation into the reaction flask under stirring, and the dropwise addition time ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com