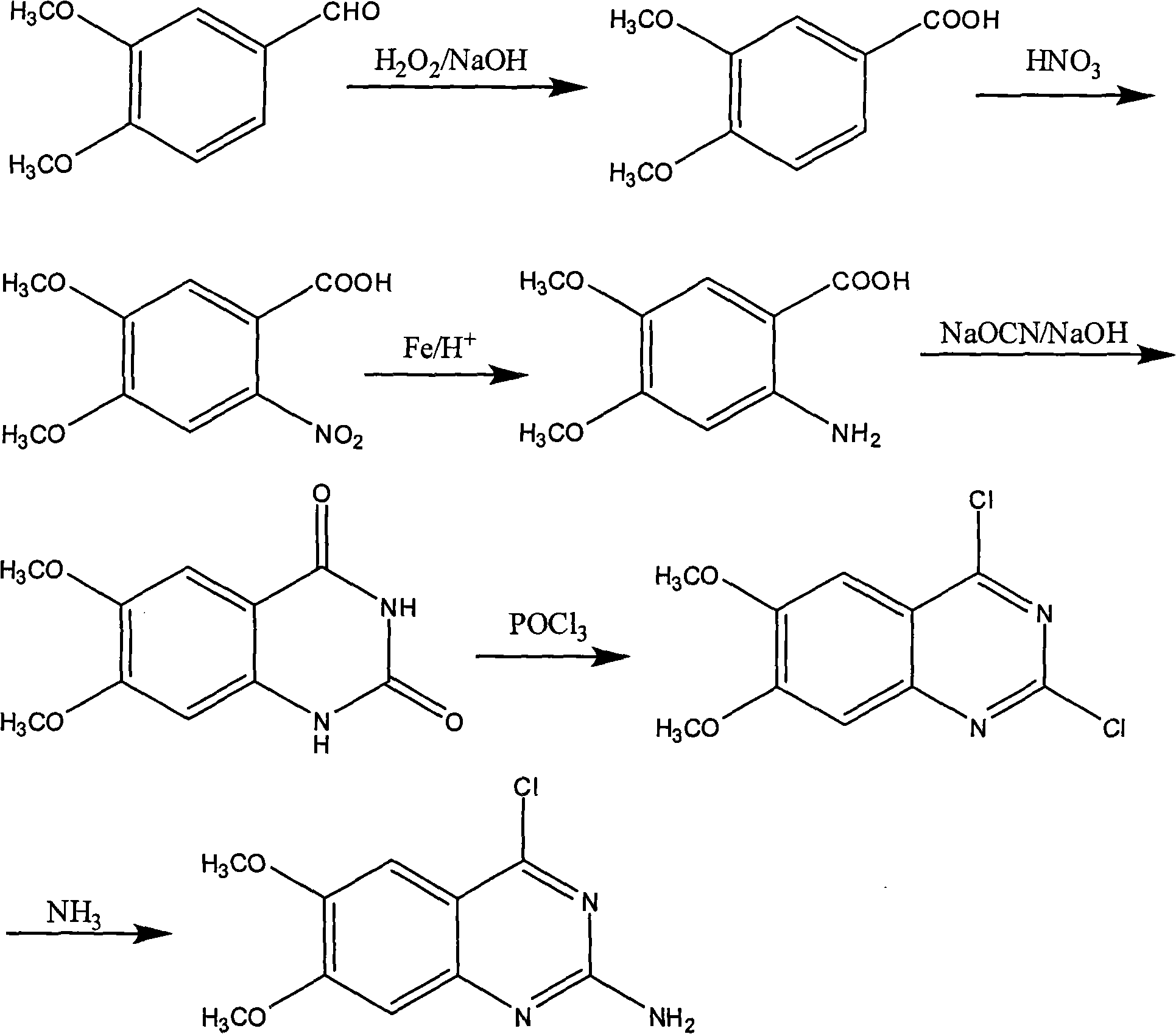
Preparation of 2-chlorin-4-amido-6,7-dimethoxy quinazoline
A technology of dimethoxyquinazoline and dimethoxy, applied in the field of chemical synthesis preparation, can solve the problems of increasing difficulty, increasing pollution of three wastes, high price, etc., and achieves reduction of production cost, pollution of three wastes, and protection of health. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) With 3,4-dimethoxybenzaldehyde as the starting material, the molar ratio of 3,4-dimethoxybenzaldehyde:hydrogen peroxide is 1:5. Dissolve 3,4-dimethoxybenzaldehyde in an alkaline solution with a concentration of 5%, stir and heat up to 20°C, and then add hydrogen peroxide with a concentration of 1% to react for 2 hours, and the 3,4-dimethoxybenzaldehyde Oxidized to 3,4-dimethoxybenzoic acid, filtered and dried to obtain 3,4-dimethoxybenzoic acid solid;
[0026] (2) According to the molar ratio of 3,4-dimethoxybenzoic acid: nitric acid is 1: 1.5. Dissolve 3,4-dimethoxybenzoic acid solid in chloroform, cool to 15°C, add nitric acid with a concentration of 65% to react with 3,4-dimethoxybenzoic acid for 2 hours, filter and dry to obtain 4,5-Dimethoxy-2-nitrobenzoic acid solid;
[0027] (3) According to the molar ratio of 4,5-dimethoxy-2-nitrobenzoic acid: iron powder: hydrochloric acid is 1: 1.5: 0.1. Add 4,5-dimethoxy-2-nitrobenzoic acid solid, iron powder, and 1% h...
Embodiment 2
[0032](1) With 3,4-dimethoxybenzaldehyde as the starting material, the molar ratio of 3,4-dimethoxybenzaldehyde:hydrogen peroxide:potassium hydroxide is 1:15:2.5. Dissolve 3,4-dimethoxybenzaldehyde in potassium hydroxide solution with a concentration of 30%, stir and heat up to 60°C, then add hydrogen peroxide with a concentration of 50% and react for 10 hours, and 3,4-dimethoxybenzene Formaldehyde is oxidized to 3,4-dimethoxybenzoic acid, filtered and dried to obtain 3,4-dimethoxybenzoic acid solid;
[0033] (2) According to the molar ratio of 3,4-dimethoxybenzoic acid: nitric acid is 1:5. Dissolve 3,4-dimethoxybenzoic acid solid in chloroform, cool to 50°C, add nitric acid with a concentration of 97% to react with 3,4-dimethoxybenzoic acid for 10 hours, filter and dry to obtain 4,5-Dimethoxy-2-nitrobenzoic acid solid;
[0034] (3) According to the molar ratio of 4,5-dimethoxy-2-nitrobenzoic acid: iron powder: hydrochloric acid is 1:7:2. Add 4,5-dimethoxy-2-nitrobenzoic ac...
Embodiment 3
[0039] (1) With 3,4-dimethoxybenzaldehyde as the starting material, the molar ratio of 3,4-dimethoxybenzaldehyde:hydrogen peroxide:sodium hydroxide is 1:10:1.5. Dissolve 3,4-dimethoxybenzaldehyde in a sodium hydroxide solution with a concentration of 15%, stir and heat up to 40°C, then add hydrogen peroxide with a concentration of 25% and react for 6 hours, and the 3,4-dimethoxybenzene Formaldehyde is oxidized to 3,4-dimethoxybenzoic acid, filtered and dried to obtain solid 3,4-dimethoxybenzoic acid;
[0040] (2) According to the molar ratio of 3,4-dimethoxybenzoic acid: nitric acid is 1:3. Dissolve 3,4-dimethoxybenzoic acid solid in chloroform, cool to 30°C, add nitric acid with a concentration of 80% to react with 3,4-dimethoxybenzoic acid for 6 hours, filter and dry to obtain 4,5-Dimethoxy-2-nitrobenzoic acid solid;
[0041] (3) According to the molar ratio of 4,5-dimethoxy-2-nitrobenzoic acid: iron powder: hydrochloric acid is 1:4:1. Add 4,5-dimethoxy-2-nitrobenzoic aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com