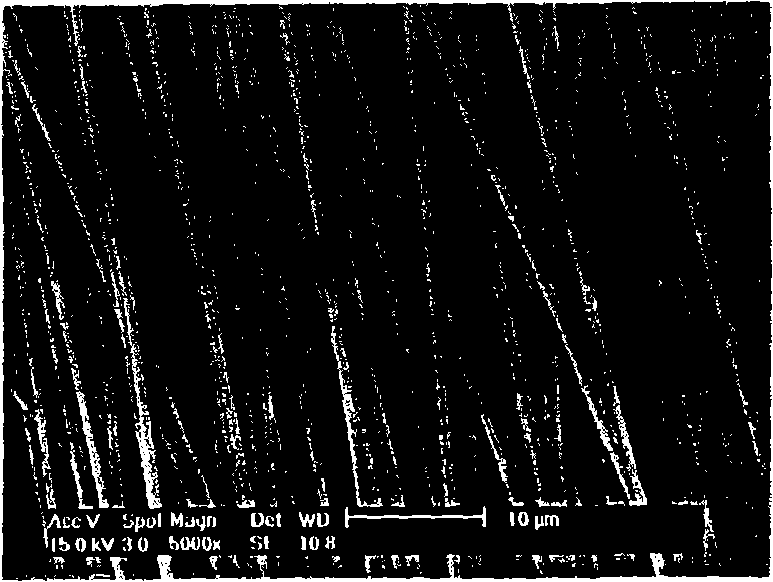
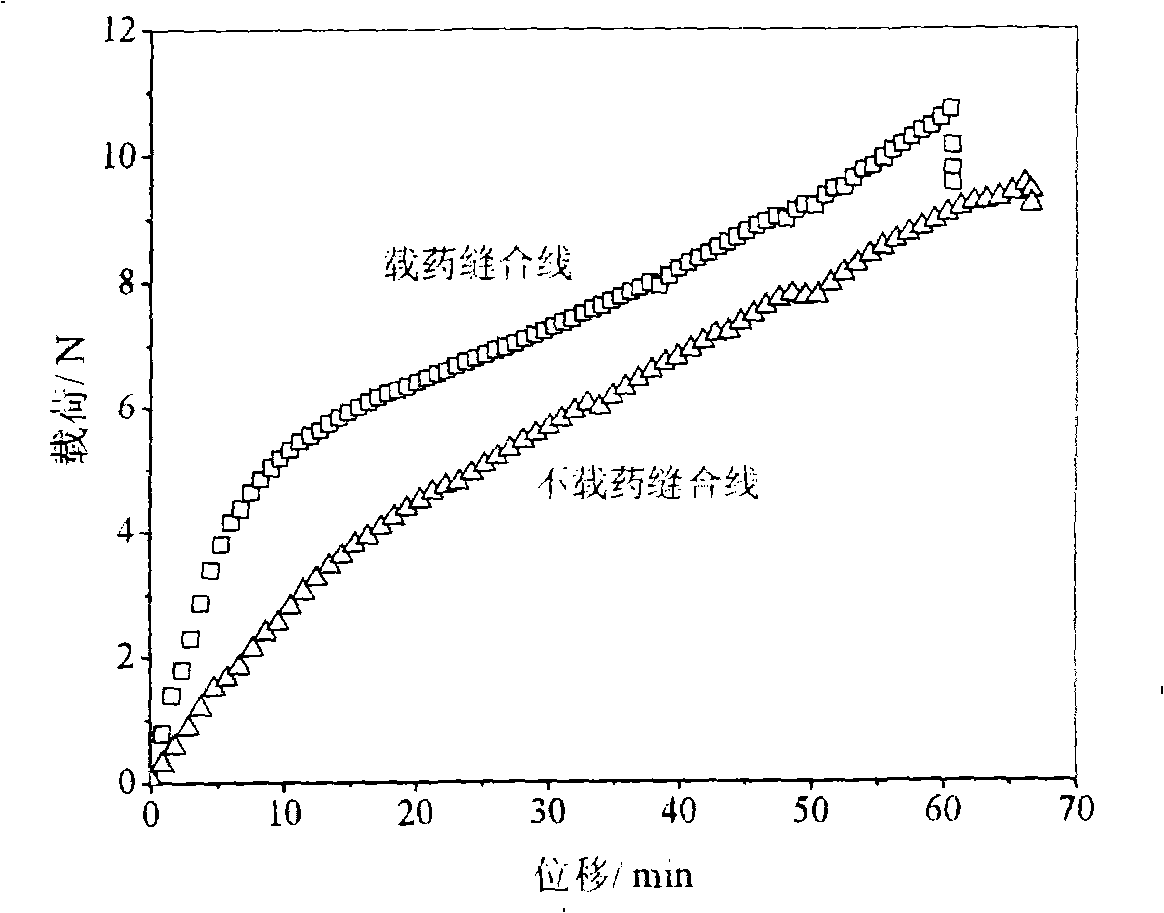
Suture thread containing bioactive components and preparation method thereof
A bioactive ingredient and suture technology, applied in the field of medical sutures and their preparation, can solve problems such as difficult controlled release of drugs, and achieve the effect of avoiding wound infection and reducing infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Preparation of sutures with releasable antibiotics that promote coagulation
[0043] (1) Preparation of raw material solution
[0044] Shell layer: The biodegradable material is L-polylactic acid (PLLA), and the solvent is trifluoroethanol. A certain mass of L-lactic acid is weighed, dissolved in a solvent to form a shell spinning solution with a concentration of 8wt%, and ultrasonically Medium shaking for 4-5 hours to obtain a transparent and uniform shell spinning solution;
[0045] Core layer: the bioactive ingredient is cefotaxime sodium, and the solvent is deionized water. Different qualities of cefotaxime sodium are weighed and dissolved in solvents respectively to make core materials with concentrations of 10wt%, 20wt%, and 30wt% for spinning solution;
[0046] Coating: biodegradable material chitosan (deacetylation degree 80%, molecular weight about 100,000), solvent is 1% acetic acid solution, chitosan is dissolved in the solvent to form a concentr...
Embodiment 2
[0058] Example 2: Preparation of sutures with the effect of releasing anticancer drugs
[0059] (1) Preparation of raw material solution
[0060] Shell layer: the biodegradable material is polycaprolactone (PCL), the solvent is chloroform: acetone with a volume fraction of 2:1, polycaprolactone is dissolved in the solvent, and the solution is placed in a water bath constant temperature tank, It can be dissolved in a water bath at 60°C for 2 hours, and the PCL concentration is kept at 7wt% to obtain a shell spinning solution;
[0061] Core material: the bioactive component is resveratrol (RT), and the solvent is absolute ethanol. Resveratrol (RT) is dissolved in the solvent, dissolved under magnetic stirring, and the control concentration is 10wt%, and the core material is obtained spinning solution;
[0062] Coating: The solute of the coating is lactic acid / glycolic acid copolymer (PGLA910), and the solvent is acetone. The solute is dissolved in the solvent to prepare a solu...
Embodiment 3
[0072] Example 3: Preparation of medical sutures that can release growth factors
[0073] Fibroblast culture, subculture and identification:
[0074] Wash the bloodstained fresh skin after surgery, remove the epidermis and subcutaneous tissue, and cut it into 0.15-1mm under sterile conditions 3 Tissue pieces attached to the bottom of the culture flask, at 37°C, 5% CO 2 Culture the cells in a static incubator for 4 hours, add DMEM culture solution containing 20% FBS, 100mg / L penicillin, and 100mg / L streptomycin, continue to cultivate, and change the medium once every 4 to 6 days with DMEM containing 20% FBS , subcultured after 3-4 weeks of culture. Fibroblasts were identified by cell morphology.
[0075] (1) Preparation of raw material solution
[0076]Shell layer: The biodegradable material is lactic acid-polyglycolic acid copolymer (PLGA910), the solvent is 3:1 tetrahydrofuran and N,N-dimethylformamide, and the shell layer spinning concentration is 0.2g / ml. Shake the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com