Process for jointly producing cyanuramide, sodium carbonate and ammonium chloride by using carbamide
A joint production and ammonium chloride technology, which is applied in the field of melamine production, joint production of melamine, soda ash and ammonium chloride, can solve the problem that melamine tail gas cannot fully utilize the waste heat steam, and achieve the effect of energy saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
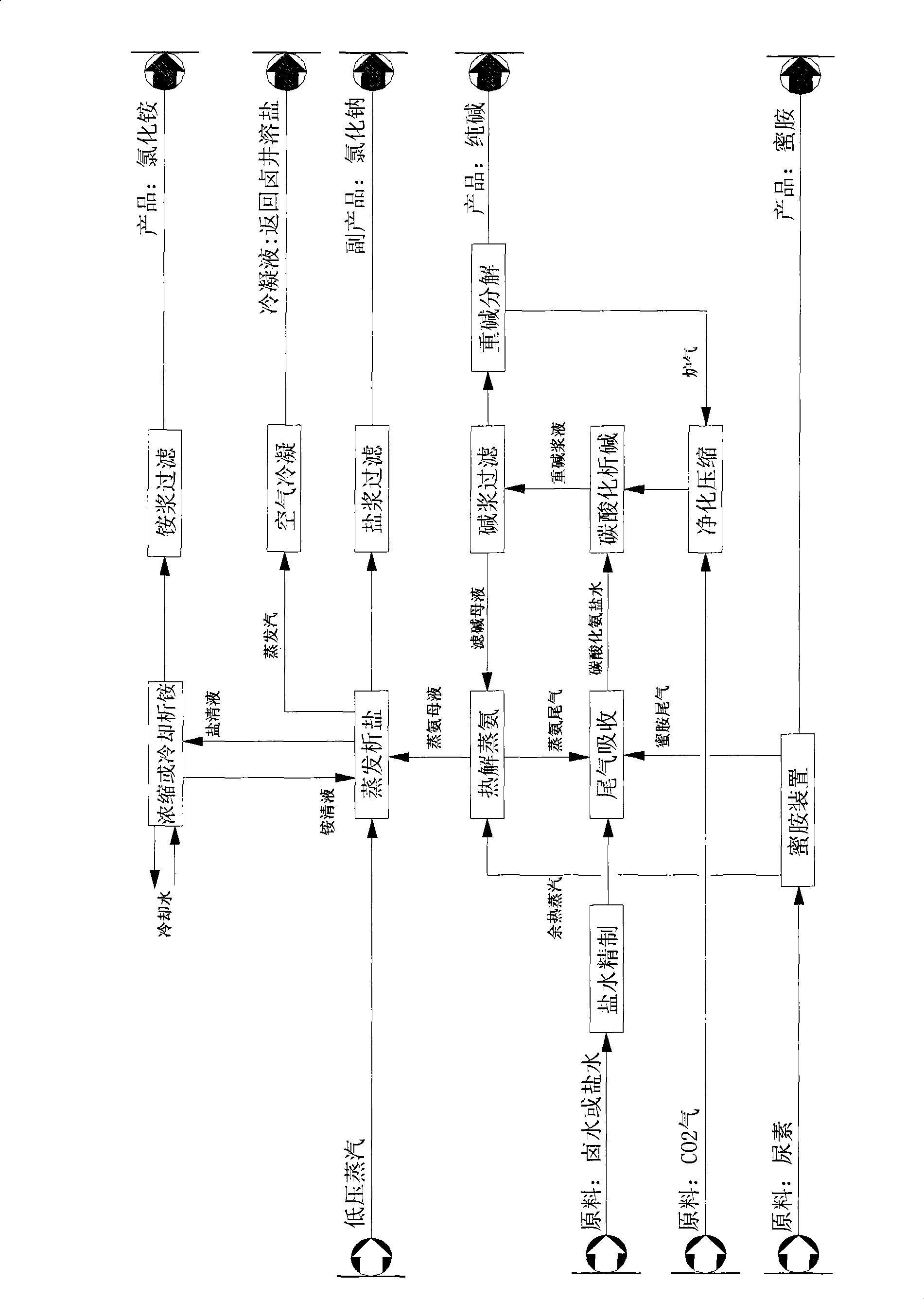
[0050] The preparation method for melamine in this embodiment is a low pressure gas phase quenching method. It selects molten urea with water content not higher than 0.5wt%, and sends it to the fluidized bed reactor by a molten urea pump, sets the reaction pressure in the fluidized bed to 0.1MPa (gauge pressure), and the reaction temperature to 390°C. Under the action of catalysts containing aluminum silicate, aluminum oxide, silicon oxide, titanium oxide, etc., urea, which accounts for 95wt% of the reactor, undergoes a series of physical and chemical reactions to generate melamine and release NH at the same time. 3 , CO 2 gas. The heat required for this reaction is indirectly provided by the high temperature molten salt in the fluidized bed reactor. The mixed gas generated by the reaction is cooled to 325°C and filtered to remove mechanical impurities such as deamination products and catalysts.
[0051] The mixed gas after high-temperature filtration enters the melamine cr...
Embodiment 2
[0059] The preparation method for melamine in this embodiment is a low pressure liquid phase quenching method. The molten urea with water content not higher than 0.5wt% is pumped to the fluidized bed reactor by the molten urea pump, the reaction pressure in the fluidized bed is set to 0.60MPa (gauge pressure), the reaction temperature is 390°C, and the Under the action of catalysts such as aluminum, aluminum oxide, silicon oxide, and titanium oxide, the urea, which accounts for more than 95% of the input into the reactor, undergoes a series of physical and chemical reactions to generate melamine and release NH at the same time. 3 , CO 2 gas. The heat required for this reaction is indirectly provided by the high temperature molten salt in the fluidized bed reactor.
[0060] The reaction product gas flowing out from the top of the reactor contains melamine, CO 2 , NH 3 , a small amount of by-products and some catalyst dust are sprayed and quenched by the overflow clear liqui...
Embodiment 3
[0070] The preparation method for melamine in this embodiment is a high-pressure liquid phase quenching method. The molten urea with water content not higher than 0.5wt% is sent to the high-pressure gas-liquid reactor by the molten urea pump, the reaction pressure is set to 8.0MPa (gauge pressure), the reaction temperature is 380°C, and the reaction does not require a catalyst, then more than 90wt% The urea produces melamine through a series of physical and chemical reactions, and releases NH at the same time 3 , CO 2 gas. The heat required for the reaction is indirectly provided by the high-temperature molten salt in the vessel.
[0071] The discharge from the top of the reactor contains melamine, NH 3 , CO 2 And a small amount of gas-liquid mixture of by-products, the gas mixture is sprayed and quenched in the urea quenching tower by the methylammonium liquid sent by the subsequent absorption tower, the temperature drops to 160 ° C, and the gas is washed by the scrubber,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com