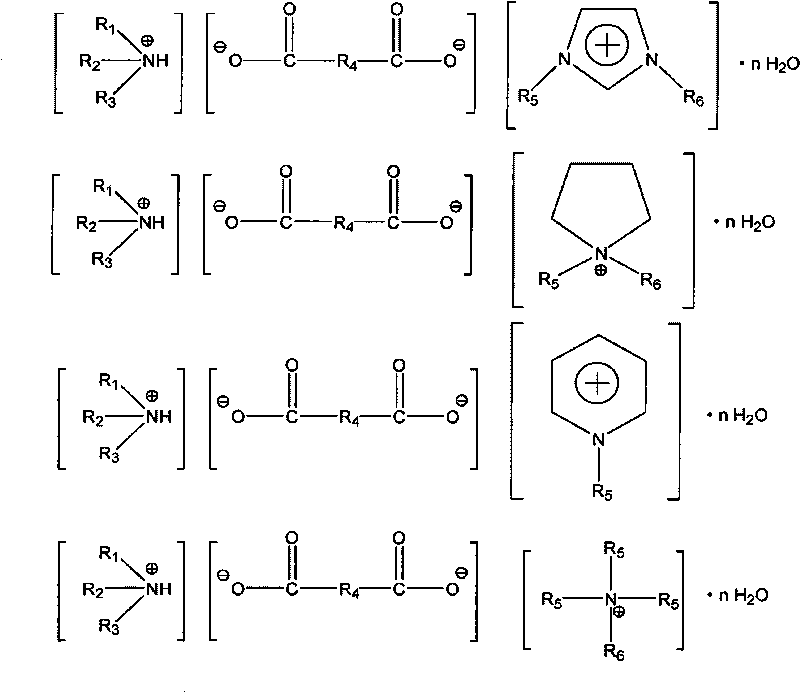
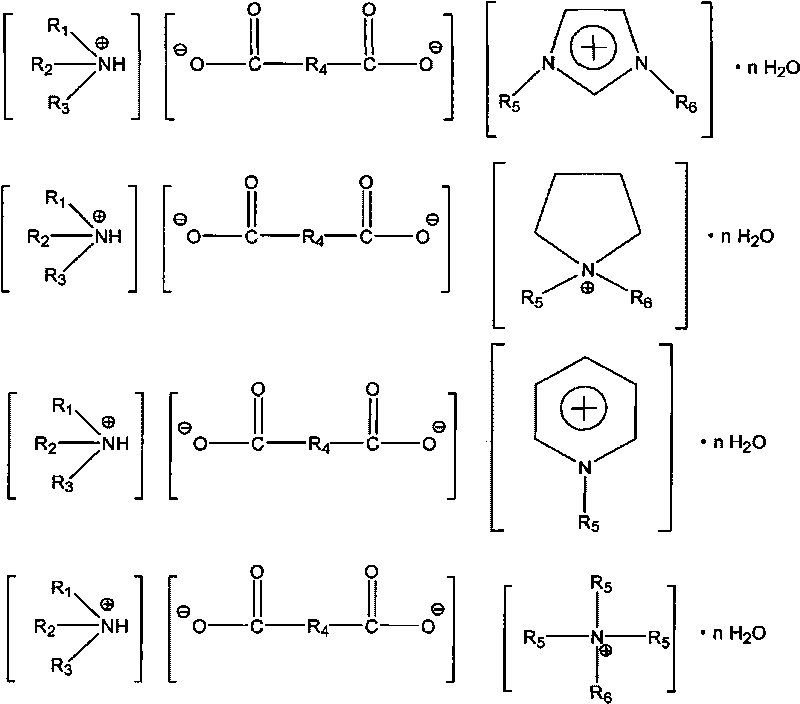
Hybrid ionic liquid hydrate, preparation process and application thereof
A technology of ionic liquids and hydrates, applied in separation methods, other chemical processes, and separation of dispersed particles, can solve problems such as poor thermal stability and oxidation resistance, high viscosity of ionic liquids, and low efficiency of mass transfer and separation. Good oxidation resistance, fast mass transfer rate, high selective absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Synthesis of the succinate hybrid ionic liquid hydrate of N, N-methylethylimidazole and diethanolamine
[0022] Step 1. Make N,N-methylethylimidazolium bromide salt into a 50% aqueous solution and place it in the reaction kettle. Under the condition of ice-water bath, slowly add Ag 2 Suspension made of O powder and water, reacted for 6 hours under stirring, stood still, filtered to remove silver bromide precipitate, and obtained 213g of N,N-methylethylimidazole alkali aqueous solution, N,N-methylethylimidazole The mass concentration of alkali is 60%, and the yield is 98.5%.
[0023] Step 2. Take 213g of N,N-methylethylimidazole base aqueous solution with a mass concentration of 60%, place it in a reaction kettle, slowly add 118g (1mol) of succinic acid dropwise at room temperature, react under stirring for 3 hours, and let it stand place.
[0024] Step 3. Add 105 g (1 mol) of diethanolamine slowly to the reaction liquid after the reaction in step 2 at room ...
Embodiment 2
[0025] Embodiment 2: Synthesis of the maleate hybrid ionic liquid hydrate of N, N-methylbutylimidazole and N-methyldiethanolamine
[0026] Step 1, with 50% concentration of N, N-methyl butyl imidazole bromide saline solution, through domestic 717 type strong basic anion exchange resin ion exchange, obtain N, N-methyl butyl imidazole alkali aqueous solution 624g, N , the mass concentration of N-methylbutylimidazole base is 25%, and the yield is 99%.
[0027] Step 2, take 624g of N,N-methylbutylimidazole alkali aqueous solution with a mass concentration of 25%, place it in a reaction kettle, slowly add 116g (1mol) of maleic acid dropwise at room temperature, react under stirring for 5 hours, and statically place.
[0028] Step 3. Add 119g (1mol) of N-methyldiethanolamine slowly dropwise to the reaction liquid after the reaction in step 2 at room temperature, and react for 12 hours under stirring. After the reaction process is completed, most of the mixture is evaporated under v...
Embodiment 3
[0029] Embodiment 3: the hydrate synthesis of the malonate hybrid ionic liquid of N, N-methylethylimidazole and tributylamine
[0030] Step 1, make N,N-methylethylimidazolium bromide into a 50% aqueous solution and place it in the reaction kettle, and slowly add Ag in a trickle under the condition of an ice-water bath 2 Suspension made of O powder and water, reacted for 8 hours under stirring, stood still, filtered to remove silver bromide precipitate, and obtained 512g of N,N-methylethylimidazole alkali aqueous solution, N,N-methylethylimidazole The mass concentration of alkali is 25%, and the yield is 98%.
[0031] Step 2. Take 512g of N,N-methylethylimidazole alkali aqueous solution with a mass concentration of 25%, put it in a 2L reaction kettle, slowly add 104g (1mol) of malonic acid dropwise at room temperature, and react for 5 hours under stirring. stand still.
[0032] Step 3. In the reaction solution after the reaction in step 2, at normal temperature, slowly add 18...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com