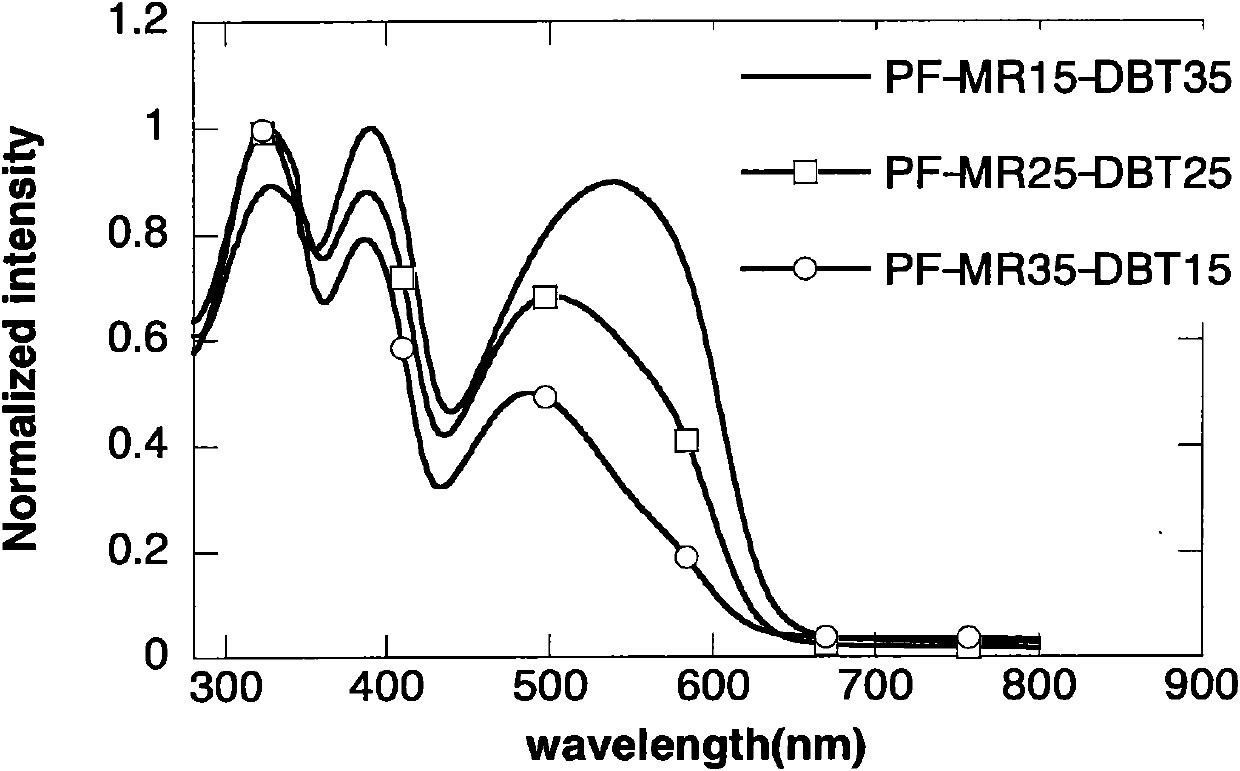
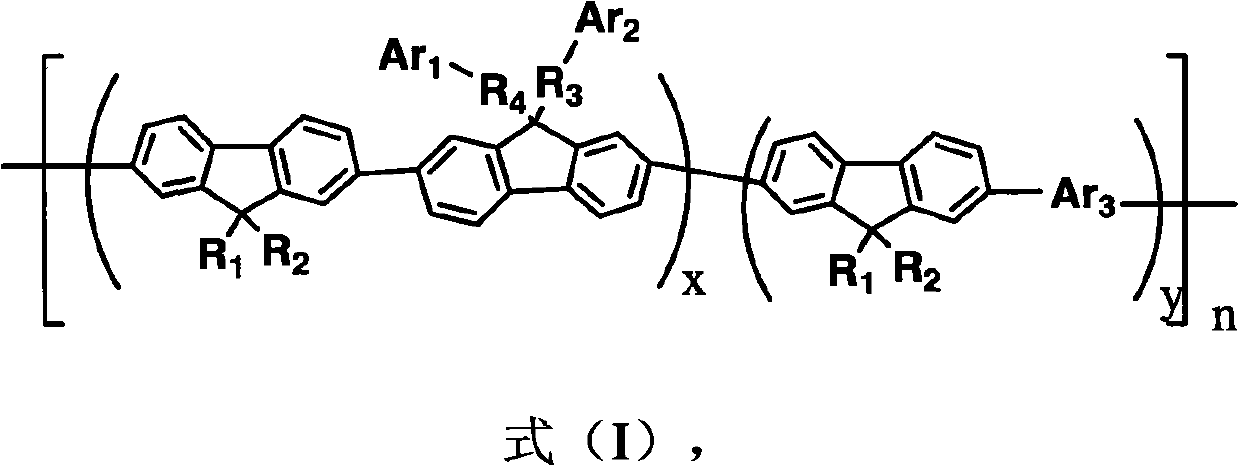
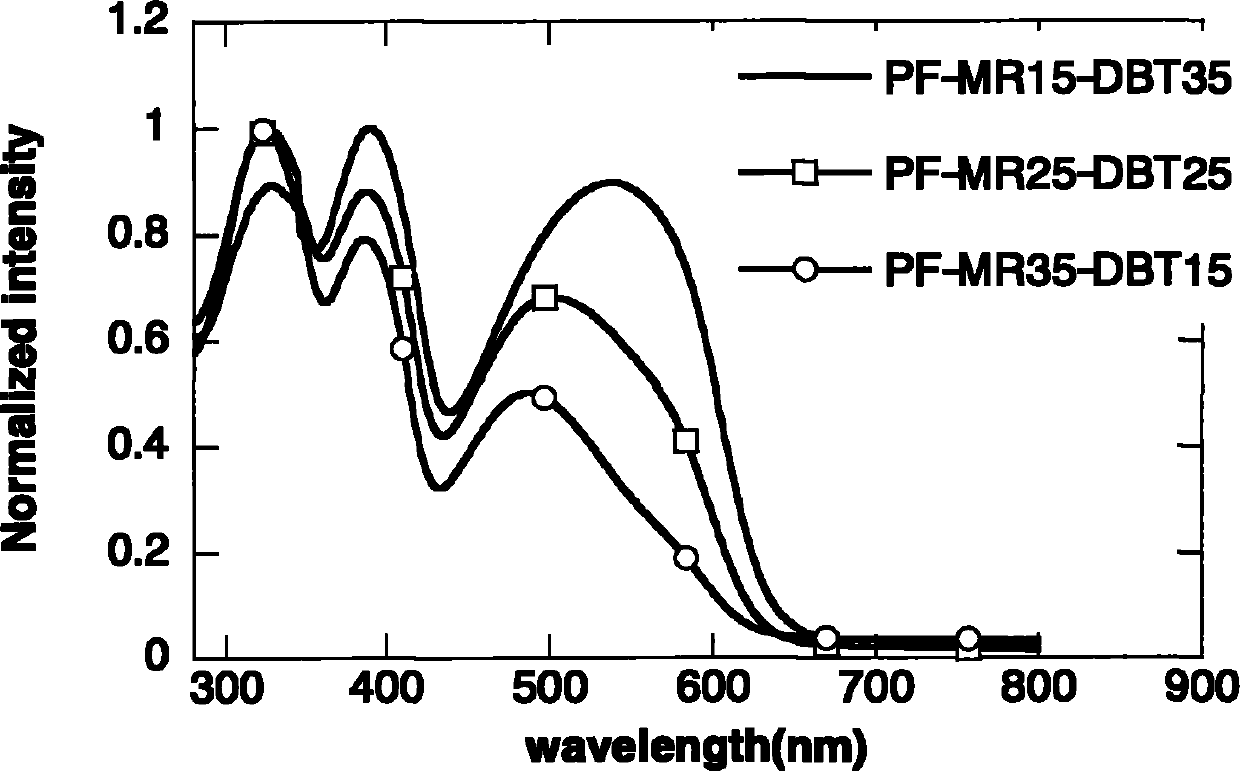
Polyfluorene conjugated polymer with thiophene and other aromatic heterocycle at C9 side chain, and preparation method and applications thereof
A technology of conjugated polymers and heterocyclic compounds, applied in the field of polyfluorene conjugated polymers and its preparation, to achieve good film-forming properties, good carrier injection and transport capabilities, and high energy conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Synthesis of 2,7-dibromo-9,9-bis-aromatic heterocyclic substituted fluorene
[0035] According to the method disclosed in Adv.Funct.Mater.2007,17,1917, with 2,7-dibromo-9,9-bis(4-triphenylamine-7-thiophene-2,1,3-benzothiadiazole The preparation of -6-hexyl)phenoxyfluorene is illustrated as an example. The synthetic route is as follows:
[0036]
[0037] (1) 4-Triphenylamine boronic acid
[0038]
[0039] In a 250ml three-necked flask, add 5g (15.4mmol) 4-bromotriphenylamine, 60mLTHF, drop 6.7mL n-BuLi (2.5M in n-hexane) when the temperature drops to -78°C, and stir at -78°C after the dropwise addition 1h, then add 23.1mmol trimethyl borate, rise to room temperature and react overnight, then add 10mL of 2M hydrochloric acid, stir for 5h, the reaction solution is extracted with ether, the organic layer is washed with anhydrous MgSO 4 After drying, the solvent was drained and the petroleum ether / silica gel column chromatography was used to obtain a white...
Embodiment 2
[0069] According to the method disclosed in J.Mater.Chem., 2002,12,2887, the synthesis of 4,7-bis(5-bromo-2-thiophene)-2,1,3-benzothiadiazole is For example, the synthesis route is shown in the figure below:
[0070]
[0071] (1) Synthesis of 3-butyl-2-phenostannane
[0072]
[0073] In a 250ml three-neck flask, add 5ml thiophene and 30ml THF, cool down to -30°C with liquid nitrogen / isopropanol, add 1.6M n-butyllithium dropwise, then react at -30°C for 30min, then add SnBu dropwise 3 Cl, react at -30°C for 2h. Extract with ether, anhydrous MgSO 4 Dry, remove the solvent under reduced pressure, and use neutral Al 2 o 3 / petroleum ether (30-60°C) column chromatography to obtain a colorless and transparent oily liquid. Yield: 72%. 1 H NMR (CDCl 3 , 500 MHz, ppm) 7.71 (1H, dd) 7.32 (1H, dd), 7.27 (1H, dd), 0.96-1.74 (27H, m, 3×Bu).
[0074] (2) Synthesis of 4,7-dithiophen-2-yl-2,1,3-benzothiadiazole
[0075]
[0076] In a 250ml three-necked flask, add 2g 4,7-di...
Embodiment 32
[0080] Example 32, Preparation of 7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-diyl)-9,9-dioctylfluorene
[0081]
[0082] Synthesize 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-diyl)-9,9 according to the method disclosed in document Macromolecules, 1997,30,7686 -Dioctylfluorene.
[0083] (1) Synthesis of 2,7-dibromo-9,9-dioctylfluorene
[0084]
[0085] Add 15.0g (0.048mol) 2,7-dibromofluorene and 200ml dimethyl sulfoxide into a three-necked flask equipped with a stirrer, and add 0.09g (0.39mol) tetrabutylammonium bromide, 15ml 50% sodium hydroxide solution and 27.8g (0.144mol) 1-bromooctane were reacted at room temperature for 3h, water and ether were added to the reactant, the organic layer was separated, washed with saturated brine, and dried over anhydrous magnesium sulfate. Filtrate, evaporate the solvent, remove the solvent diethyl ether, pass through a column with silica gel / petroleum ether, and separate the desired components. Yield: 88%. m.p.: 52-54°C....
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com