Method for preparing protein chip by in-situ synthesis
A technology of protein chip and in-situ synthesis, which is applied in the process of producing decorative surface effects, manufacturing microstructure devices, decorative arts, etc., and can solve the problems of difficult control of experimental conditions, insufficient repeatability of experimental results, and cumbersome manufacturing processes, etc. problems, to avoid the uncertainty of protein conformation and orientation, and to avoid surface non-specific adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of aniline derivative monomers with biologically active units
[0030] Dissolve biotin glycol phosphoramidite in anhydrous acetonitrile, then add anhydrous acetonitrile solution of aniline-o-methanol, wherein: the molar ratio of biotin glycol phosphoramidite to aniline-o-methanol is 10:1~1:10 ; Add an appropriate amount of 5-ethylthiotetrazolium (ETT) activator, so that the concentration of the formed activator anhydrous acetonitrile solution is 0.25mol / L; after stirring at room temperature for 30 minutes, carry out vacuum distillation to powder; Add excess oxidation solution, the oxidation solution is formed by dissolving elemental iodine in tetrahydrofuran: water: triethylamine = 8:1:1 mixed solvent, the mass percent concentration of the solution is 4%; after stirring at room temperature for 10 minutes, Reduce to neutrality with sodium thiosulfate, then distill THF under reduced pressure, extract with chloroform, dry the organic layer over anhyd...
Embodiment 2
[0033] Example 2: Preparation of protein chips
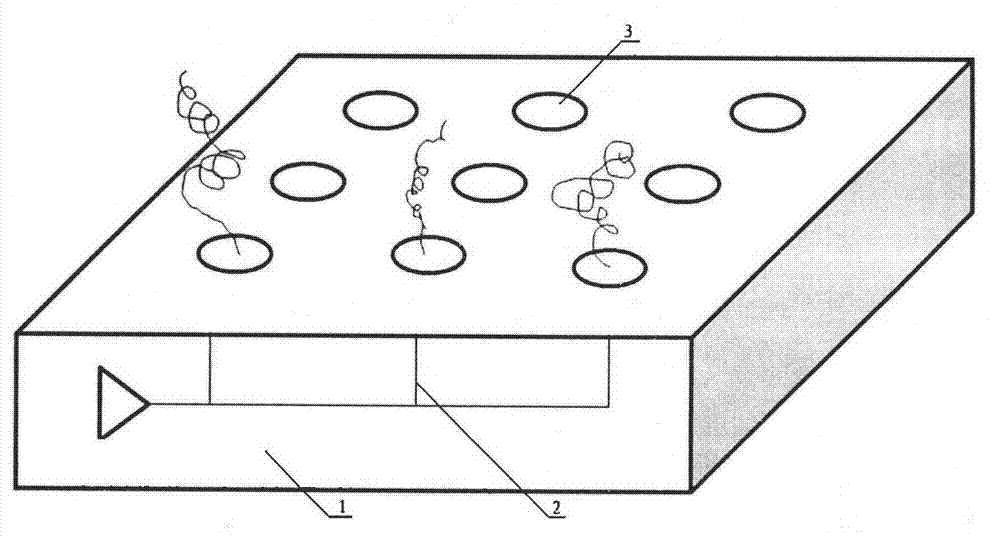
[0034] Build microelectrode arrays on individually addressable logic circuit chips (see figure 1 shown), each electrode on this circuit is connected by a switch of a complementary metal oxide semiconductor (CMOS) transistor, by sending an electronic address signal to the common node circuit and then to the SRAM (static random access memory) associated with each electrode body) to turn on the switch; the microelectrode array is placed in a specially designed fluid reactor, and the computer digitally instructs the microelectrode array to respond to digital commands to assemble the conductive polymer; the reaction liquid in the reactor is prepared with The aniline monomer of the biotin substituent is mixed with 1 to 10 times the molar amount of aniline and an appropriate amount of potassium chloride as the electrolyte; under the control of the logic circuit, the pulse is 10 milliseconds on and 10 milliseconds off, at 0.5 to 10 mill...
Embodiment 3
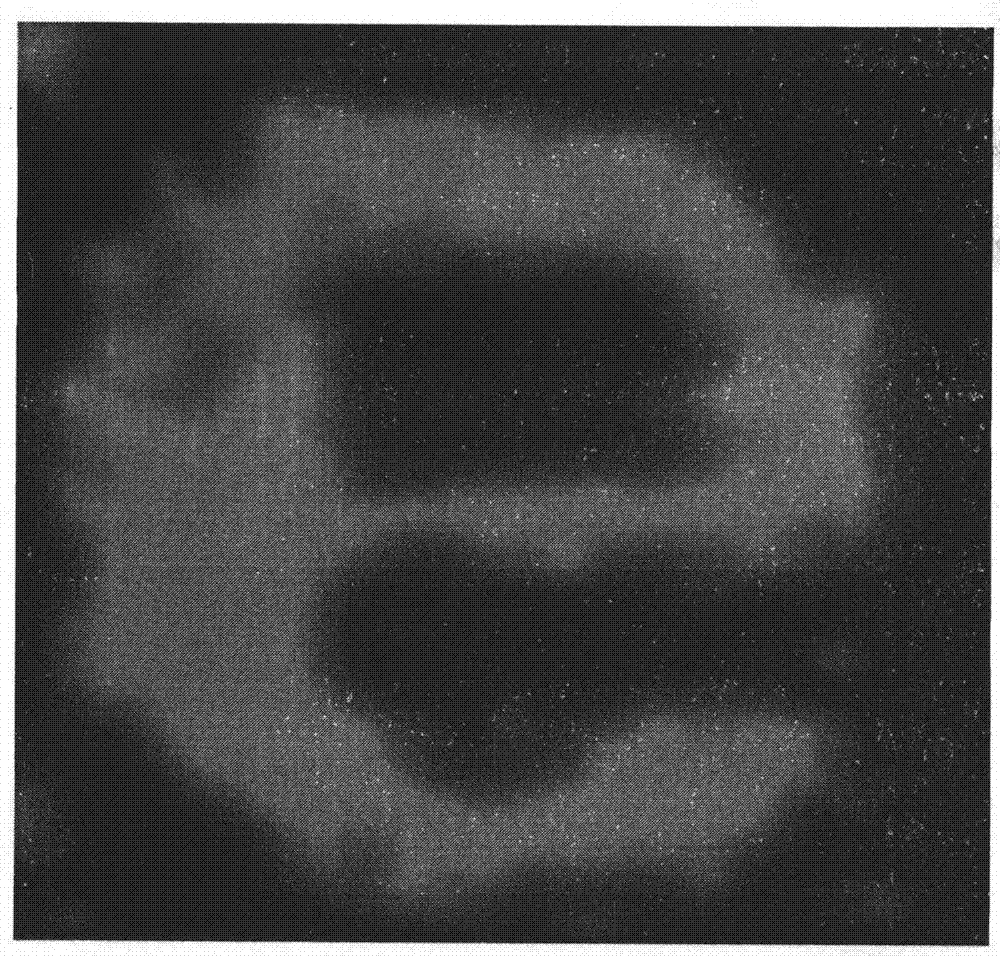
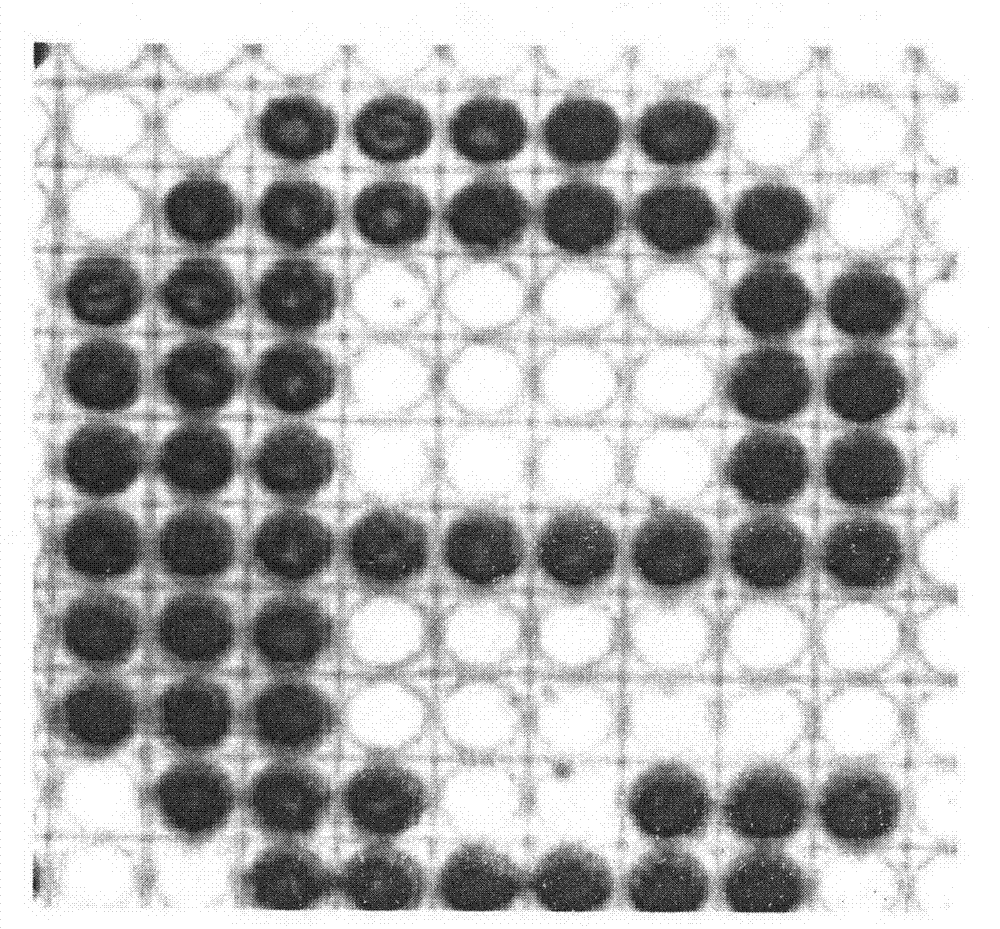
[0037] Embodiment 3: Demonstrate the relation experiment of electric current density and reaction time and the degree of polymerization of conductive polyaniline
[0038] The positive charge is passed on the electrode at different pulse intervals, and the total charge is determined by the integral of the pulse and time. For number 1, the pulse is on for 2 milliseconds and off for 10 milliseconds; for number 2, the pulse is on for 4 milliseconds and off for 10 milliseconds; that is, for each next number, the pulse on time is doubled, and the total time remains unchanged for 60 seconds , the voltage is always maintained at 1.0 volts, as shown in Table 1.
[0039] Table 1 Correspondence between numbers and pulses
[0040] number
[0041] The electrodes were rinsed with deionized water and then blown dry with nitrogen, and then optical measurements were performed. Such as Figure 4 As shown, the number 1 is barely visible, the number 2 is gradually clear, and the num...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com