Application of benzo-phenylpropanoids in preparing drug for treating viral hepatitis B
A technology of benzophenanil and its application, which is applied in the application field of benzophenanil for preparing a drug for the treatment of viral hepatitis B, and can solve the problems such as the anti-HBV activity of benzophenazone compounds that are not reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
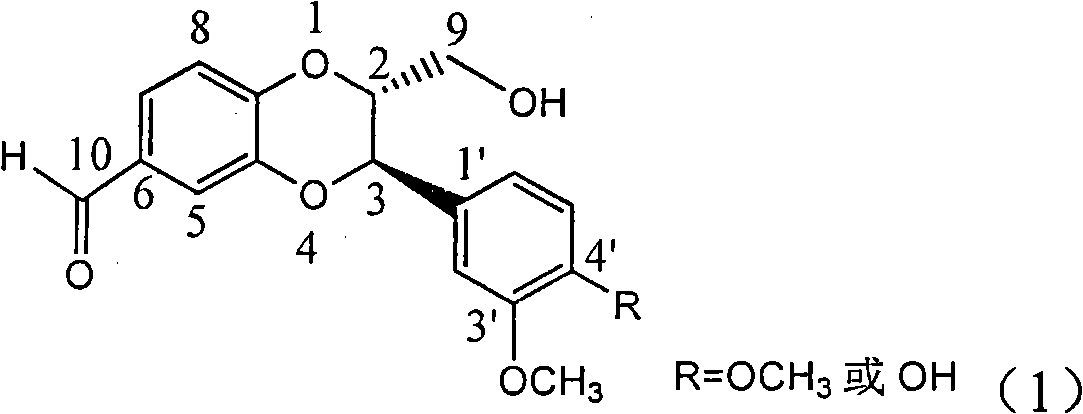
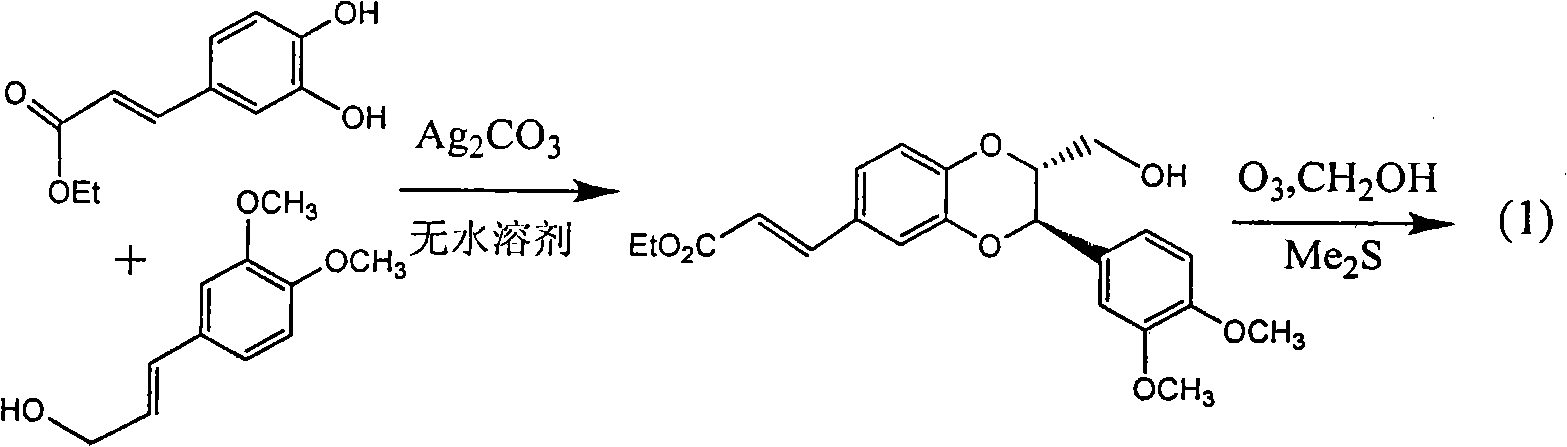
[0026] Example 1: Compound (±)-2,3-trans-3-(3,4-Dimethoxyphenyl-2-hydroxymethyl-2,3-dihydrobenzo[b][1,4]dioxane Preparation of cyclo-6-yl-formaldehyde
[0027] Instruments and reagents:
[0028] UV spectrum was measured with Shimadzu UV-240 UV spectrophotometer; proton nuclear magnetic resonance spectrum 1 H-NMR is measured by INOVA superconducting nuclear magnetic resonance spectrometer (VARIAN INOVA-400MHz) (tetramethylsilyl ether TMS is the internal standard); ESI-MS is measured by BrukerEsquire 3000+ mass spectrometer, column chromatography uses silica gel (100-200, 200-300 and 300-400 mesh) and thin layer chromatography silica gel GF254 (10-40 mesh) are produced by Qingdao Ocean Chemical Plant; all reagents used are analytical pure, thin layer preparative chromatography (PTLC) ) Use Merck's aluminum foil silica gel plate; Sephadex LH-20 for column chromatography uses the product of Amersham Pharmacia Biotech AB in Sweden; reverse-phase silica gel RP-18 uses the Chromatorex pr...
Embodiment 2
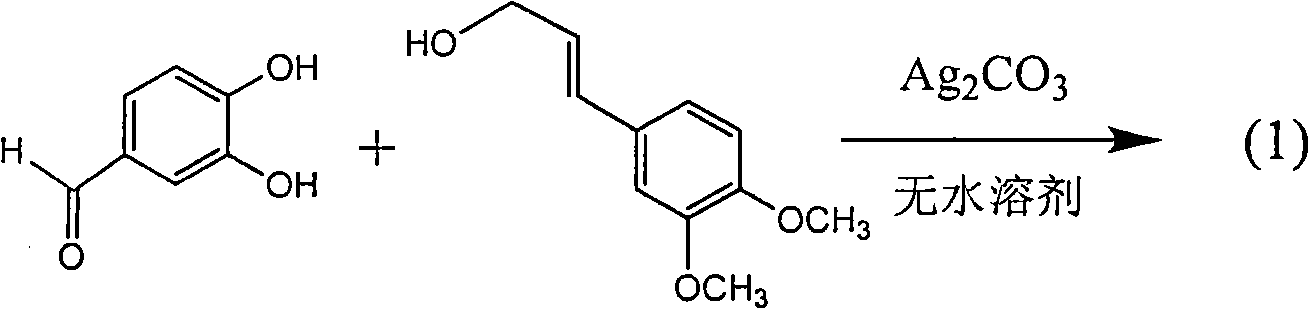
[0034] Example 2: Compound (±)-2,3-trans-3-(3-methoxy-4-hydroxyphenyl)-2-hydroxymethyl-2,3-dihydrobenzo[b][1,4] two Preparation of oxane-6-yl-formaldehyde
[0035] Apparatus and reagents: the same as in Example 1.
[0036] 2.1 Preparation method 1 (direct oxidation coupling method under silver salt catalysis):
[0037] In a dry 500 ml three-necked flask, add 0.38 g of 3,4-dihydroxybenzaldehyde and 0.52 g of coniferyl alcohol, and add 80 ml of anhydrous benzene and 20 ml of anhydrous acetone under argon protection. Stir at 60°C for 20 minutes, then add 0.765 g of silver carbonate and stir vigorously for 7 hours. The mixture was filtered, the filtrate was concentrated under reduced pressure, and the sample was mixed with silica gel, followed by 16 g of 200-300 mesh silica gel column chromatography, and repeated elution with petroleum ether: ethyl acetate (3:1) to obtain 0.328 g of a yellow solid. The yield was 38. %. R f (Chloroform / methanol=25:1): 0.32; proton nuclear magnetic re...
Embodiment 3
[0041] Example 3: (±)-2,3-trans-3-(3,4-Dimethoxyphenyl)2-hydroxymethyl-2,3-dihydrobenzo[b][1,4]dioxane -6-yl-formaldehyde inhibits the replication of hepatitis B virus deoxyribonucleic acid (HBV DNA) secreted by HepG2.2.15 cells
[0042] 3.1 Cell culture:
[0043] HepG2.2.15 cells were cultured in DMEM medium containing 10% inactivated fetal bovine serum, 100U / ml penicillin and 100U / ml streptomycin, 100μg / ml G418, and placed at 37°C, 5% CO 2 , Cultivate in an incubator with 100% relative humidity.
[0044] 3.2 Determination of compound (±)-2,3-trans-3-(3,4-dimethoxyphenyl)2-hydroxymethyl-2,3-dihydrobenzo[b][1, using MTT method 4] The inhibitory effect of dioxane 6-yl-formaldehyde on the growth of HepG2.2.15 cells:
[0045] Take HepG2.2.15 cells in logarithmic growth phase and dilute the cells to 1×10 with medium 5 Pcs / ml, seeded on 96-well cell culture plate, 100 microliters per well, at 37℃, 5% CO 2 After culturing in a 100% relative humidity incubator for 24 hours, add the compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com