Method for one-step synthesis of diphenylenediamine derivatives through oxidative coupling reaction of phenylamine derivatives
A technology of aniline derivatives and benzphenyl diamine, which is applied in the field of synthesis of benzphenyl diamine derivatives, can solve the problems of pneumoconiosis and harm to human health in contacts, and achieve good product quality, low cost and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
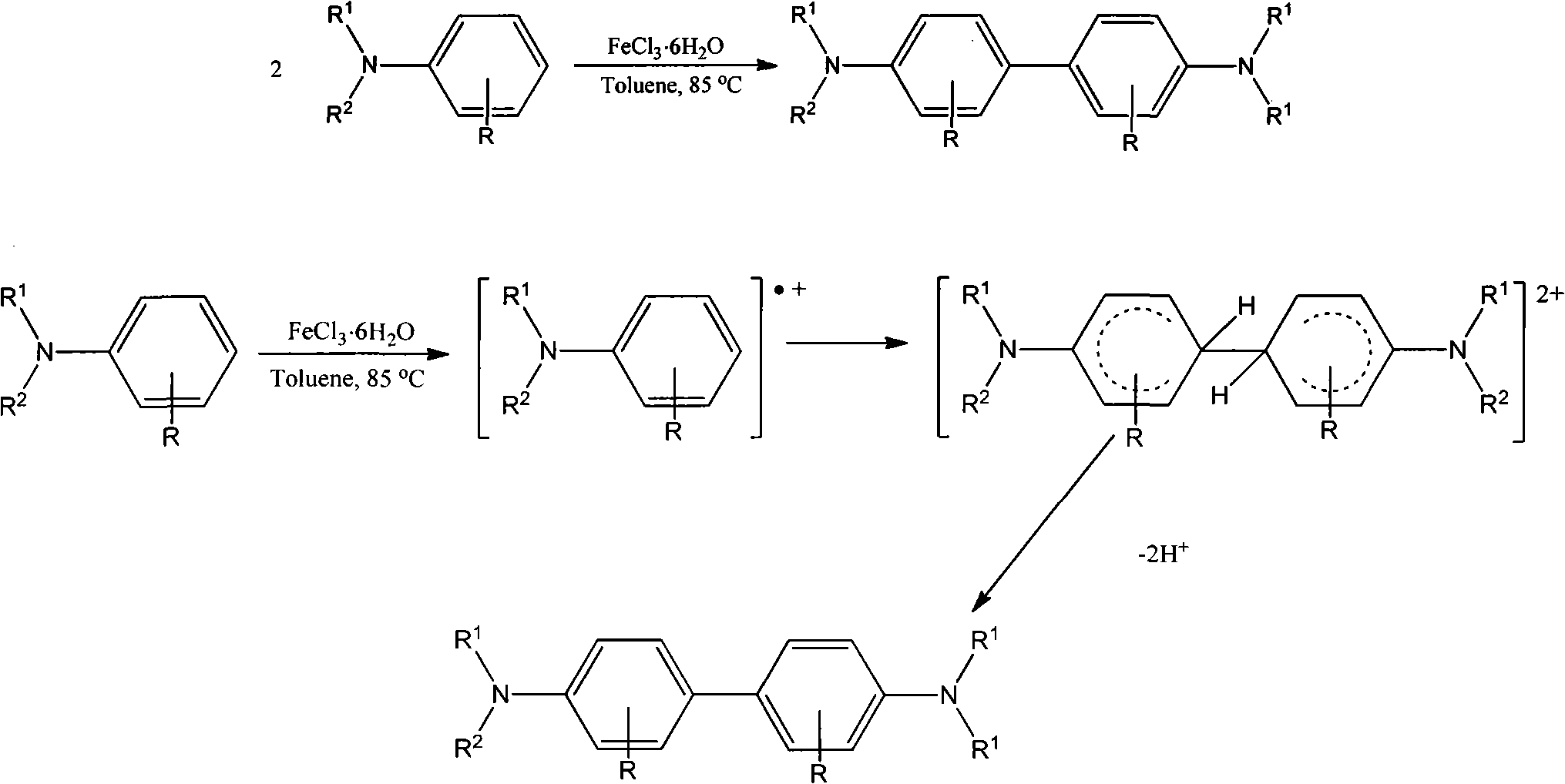
[0029] A method for synthesizing biphenyldiamine derivatives in one step through oxidative coupling of aniline derivatives, the specific steps are as follows:
[0030] (1) With N, N-dibenzylaniline as raw material, ferric chloride hexahydrate as oxidant, and toluene as solvent, according to N, N-dibenzylaniline (mmol): ferric chloride hexahydrate (mmol): toluene (milliliter) ratio is the ratio of 1: 2.5: 5, in reactor, add iron trichloride hexahydrate (0.2703g, 1mmol) oxidant and toluene solvent (2mL) earlier, add N under stirring , N-dibenzylaniline (0.1093g, 0.4mmol), after the addition was completed, the temperature was raised to 85°C, and the coupling reaction was carried out with continuous stirring for 2 hours.
[0031] (2) After step (1) is completed, the reaction solution of the biphenylenediamine derivative prepared in step (1) was cooled naturally in air, and ammonia water (10 mL) was added to completely terminate the reaction. Extract with 10mL of dichloromethane e...
Embodiment 2
[0034] A method for synthesizing biphenyldiamine derivatives in one step through oxidative coupling of aniline derivatives, the specific steps are the same as in Example 1, wherein:
[0035] In step (1), N, N-dimethylaniline is used as raw material, ferric chloride hexahydrate is used as oxidant, and toluene is used as solvent, according to N, N-dimethylaniline (mmol): three Ferric chloride (mmol): toluene (milliliter) ratio is the ratio of 1: 2.5: 5, in reactor, add iron trichloride hexahydrate (0.2703g, 1mmol) oxidant and toluene solvent (2mL) earlier, stir Additional N,N-dimethylaniline (50.7 μL, 0.4 mmol) was added.
[0036] In step (2), 10 mL of saturated potassium carbonate solution was added to quench the reaction, and the eluent was a mixture of ethyl acetate: petroleum ether with a volume ratio of 1:70 to obtain a light yellow needle-like solid product N, N, N' , N'-tetramethyl-4,4'-diaminobiphenyl (37.0 mg, yield 77%).
Embodiment 3
[0038] A method for synthesizing biphenyldiamine derivatives in one step through oxidative coupling of aniline derivatives, the specific steps are the same as in Example 1, wherein:
[0039] In step (1), N, N-diethylaniline is used as raw material, iron trichloride hexahydrate is used as oxidant, and toluene is used as solvent, according to N, N-diethylaniline (mmol): trihydrate hexahydrate Ferric chloride (mmol): toluene (milliliter) ratio is the ratio of 1: 2.5: 1, in reactor, add ferric chloride hexahydrate (1.3515g, 5mmol) and toluene (2mL) earlier, add under stirring N,N-Diethylaniline (320.9 μL, 2 mmol).
[0040] In step (2), 20 mL of saturated potassium carbonate solution was added to quench the reaction, and the eluent was ethyl acetate: petroleum ether with a volume ratio of 1: 100 mixed solution to obtain white solid product N, N, N', N' - Tetraethyl-4,4'-diaminobiphenyl (65.7 mg, yield 22%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com