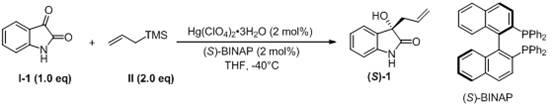
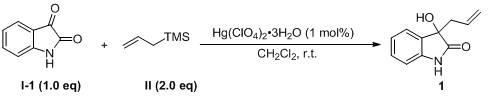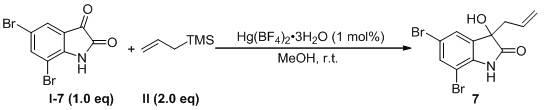Method for preparing 3-allyl-3-hydroxy oxindole
A technology of hydroxyl oxidation and allyl group, applied in the direction of organic chemistry, etc., can solve the problems of narrow application range of substrates, limited reaction substrates, and difficult source of raw materials, etc., to achieve convenient post-processing procedures, convenient recycling, and low price Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 3-allyl-3-hydroxyindole 1 Synthesis:
[0039]
[0040] In a 10.0 mL reaction flask, add mercury(II) trihydrate perchlorate [Hg(ClO 4 ) 2 ·3H 2 O, 1.8 mg, 0.004 mmol], isatin I-1 (58.8 mg, 0.40 mmol), anhydrous dichloromethane (2.0 mL), allyltrimethylsilane Ⅱ (127.0 mu L, 0.80 mmol), stirred at room temperature, TLC detected that the raw materials had basically reacted, stopped the reaction, transferred the reaction solution to a 100 mL round bottom flask, diluted with 30 mL ethyl acetate, added 10 drops of concentrated hydrochloric acid dropwise, and continued to stir for 5- After 10 minutes, TLC point plate found that there was only one product point, the organic phase was washed once with saturated sodium bicarbonate solution, once with saturated brine, and spin-dried for column chromatography, the eluent was (acetone / dichloromethane=1 / 10), get the product 1 As a white solid 71.8 mg, yield 95%. 1 H NMR (400 MHz, DMSO- d 6 ): 10.21 (s, 1H), 7.27-7.25 (m,...
Embodiment 2
[0042] 3-allyl-3-hydroxy-5-chloroindole 2 Synthesis:
[0043]
[0044] Add mercury(II) trifluoromethanesulfonate [Hg(OTf) 2 ,2.0 mg, 0.004 mmol], 5-chloroisatin I-2 (71.2 mg, 0.40 mmol), anhydrous tetrahydrofuran (3.0 mL), allyltrimethylsilane Ⅱ (127.0 mu L, 0.80 mmol), stirred at 0°C, TLC detected that the reaction of the raw materials was basically complete, the reaction was stopped, the reaction solution was transferred to a 100 mL round bottom flask, diluted with 20 mL ethyl acetate, added dropwise with 10 drops of concentrated hydrochloric acid, and continued to stir for 5 -10 minutes, after the TLC point plate found that there was only one product point, the organic phase was washed once with saturated sodium bicarbonate solution, once with saturated brine, and spin-dried for column chromatography, the eluent was (acetone / dichloromethane=1 / 10), get the product 2 As a white solid 85.6 mg, yield 97%. 1 H NMR (400 MHz, DMSO- d 6 ): d 10.38 (s, 1H), 7.29 (s, ...
Embodiment 3
[0046] 3-allyl-3-hydroxy-5-nitrooxindole 3 Synthesis:
[0047]
[0048] Add indium(III) perchlorate octahydrate [In(ClO 4 ) 3 ·8H 2 O, 22.0 mg, 0.04 mmol], 5-nitroisatin I-3 (76.9 mg, 0.40 mmol), anhydrous chloroform (3.0 mL), allyltrimethylsilane Ⅱ (127.0 mu L, 0.80 mmol), stirred at 40°C, TLC detected that the raw materials were basically reacted, stopped the reaction, transferred the reaction solution to a 100 mL round bottom flask, diluted with 30 mL ethyl acetate, added 8 drops of concentrated hydrochloric acid, and continued to stir for 5- After 10 minutes, the TLC point board found that there was only one product point, and the organic phase was washed once with saturated sodium bicarbonate solution, once with saturated brine, and spin-dried for column chromatography, and the eluent was (acetone / dichloromethane=1 / 10), get the product 3 It is 89.0 mg of white solid, and the yield is 95%. 1 H NMR (400 MHz, DMSO- d 6): d 10.99 (s, 1H), 8.20-8.18 (m, 1H), 8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com