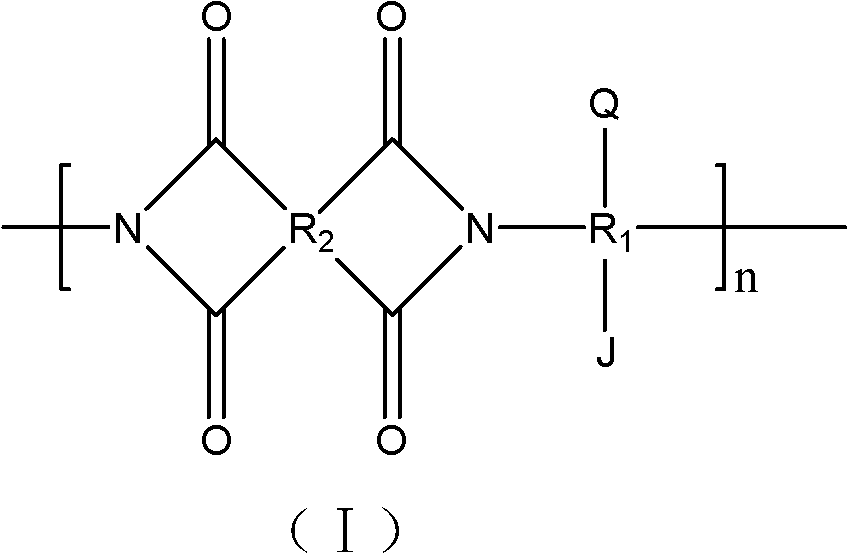
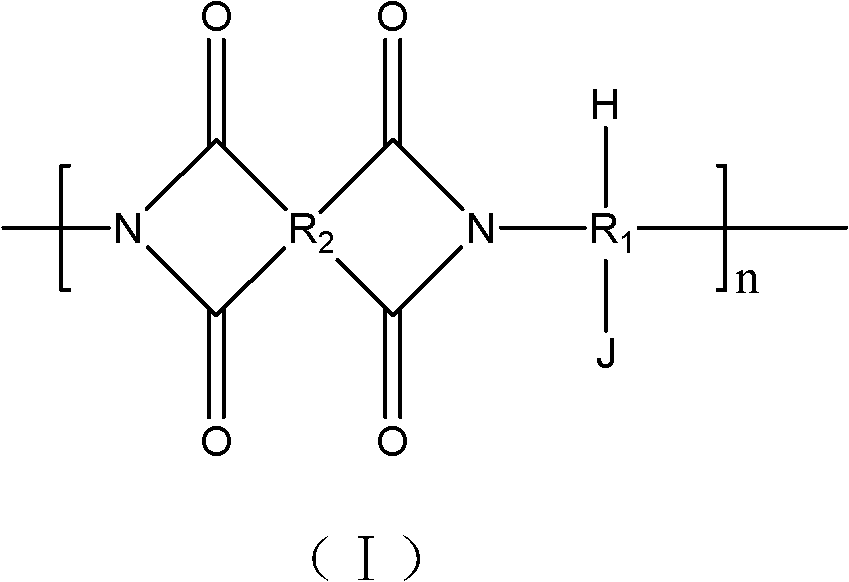
Polyimide with phosphoric acid side chain-containing long chain and preparation method and application thereof
A technology of polyimide and phosphoric acid, applied in fuel cell parts, fuel cells, electrical components, etc., can solve the problems of high vanadium ion permeability, complicated process, high production cost, etc., and achieve simple and easy preparation process , easy environment friendly, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Under nitrogen protection, after dissolving 0.02mol 4,4'-diamino-4"-hydroxytriphenylmethane in 50ml of m-cresol, polycondensation reaction with pyromellitic dianhydride at -10°C for 3 hours , then add 25ml xylene, heat up to 100°C for dehydration reaction for 3 hours, to obtain compound a; the compound
[0040] The structural formula of a is: where R 1 for R 2 for n=50; The spectral analysis of compound b is as follows: 1 H-NMR (DMSO-d6) "9.38 (s, OH, 1H), 8.35-6.74 (m, PhH, 14H), 5.69 (s, CH, 1H);
[0041] (2) Dissolve 0.01mol of compound b in 20ml of N,N-dimethylformamide, add 0.01mol of triethylamine and 0.01mol of 2-bromopropionyl bromide, and react at 0°C for 1 hour to obtain compound b; the compound The structural formula of b is: where R 1 for R 2 for n=50, R 3 for The spectral analysis of compound b is as follows: 1H-NMR (DMSO-d6) "8.35-6.74 (m, PhH, 14H), 5.69 (s, CH, 1H), 4.47 (s, CH, 1H), 1.90 (s, CH 3 ,3H);
[0042] (3) Dissolve 0....
Embodiment 2
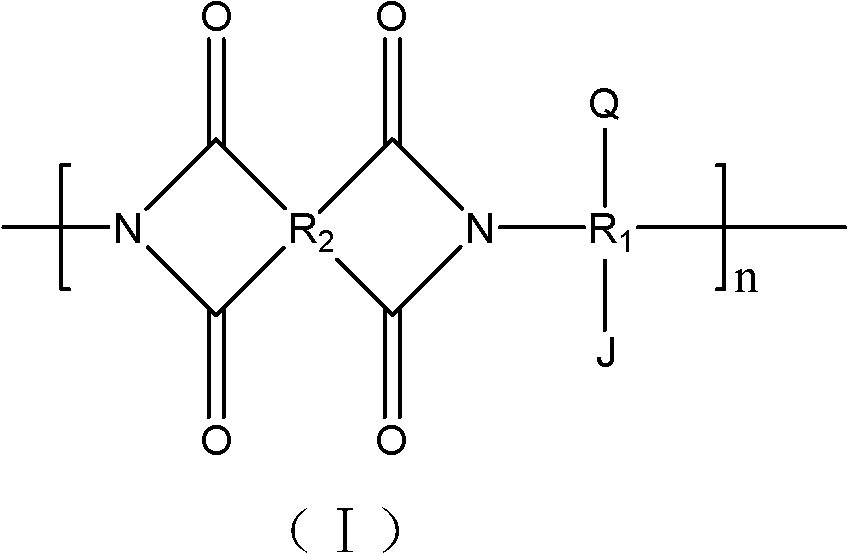
[0052] (1) Under nitrogen protection, after dissolving 0.02mol 3,5-diaminophenol in 50ml N,N-dimethylacetamide, mix with 0.028mol 3,3',4,4'-biphenyltetracarboxylic acid di The anhydride was subjected to polycondensation reaction at -5°C for 8 hours, then 25ml of xylene was added, and the temperature was raised to 120°C for dehydration reaction for 10 hours to obtain compound a; the structural formula of compound a was: where R 1 for R 2 for n=500; The spectral analysis of compound a is as follows: 1 H-NMR (DMSO-d6): 8.5~7.5 (m, PhH, 9H), 9.40 (s, OH, 2H);
[0053] (2) Dissolve 0.01mol of compound a in 30ml of dioxane, add 0.03mol of triethylamine and 0.03mol of 2-bromoisobutyryl bromide, and react at 15°C for 3 hours to obtain compound b; the structural formula of compound b is : where R 1 for R 2 for n=500, R 3 for The spectral analysis of compound b is as follows: 1 H-NMR (DMSO-d6): 8.5~7.5 (m, PhH, 9H), 1.98 (s, CH 3 ,6H);
[0054] (3) Dissolve 0.01 mo...
Embodiment 3
[0060] (1) Under nitrogen protection, after dissolving 0.03mol 3,3'-diamino-4,4'-dihydroxybiphenyl in 80ml m-cresol, mix with 0.045mol 3,3',4,4'-bis Phenyl ether tetracarboxylic dianhydride was subjected to polycondensation reaction at 0°C for 12 hours, then 40ml of xylene was added, and the temperature was raised to 170°C for dehydration reaction for 16 hours to obtain compound a; the structural formula of compound a was: where R 1 for R 2 for n=3000; The spectral analysis of compound a is as follows: 1 H-NMR (DMSO-d6) "9.35 (s, OH, 2H), 8.39-6.71 (m, PhH, 12H);
[0061] (2) Dissolving 0.02mol compound a in 50ml pyridine, adding 0.1mol 2-bromobutyryl bromide, and reacting at 30°C for 7 hours to obtain compound b; the structural formula of compound b is:
[0062] where R 1 for R 2 for n=3000, R 3 for The spectral analysis of compound b is as follows: 1 H-NMR (DMSO-d6) "8.33-6.77 (m, PhH, 12H), 5.66 (s, CH, 1H), 4.42 (s, CH, 1H), 1.08 (s, CH 3 , 3H), 2.13(m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com