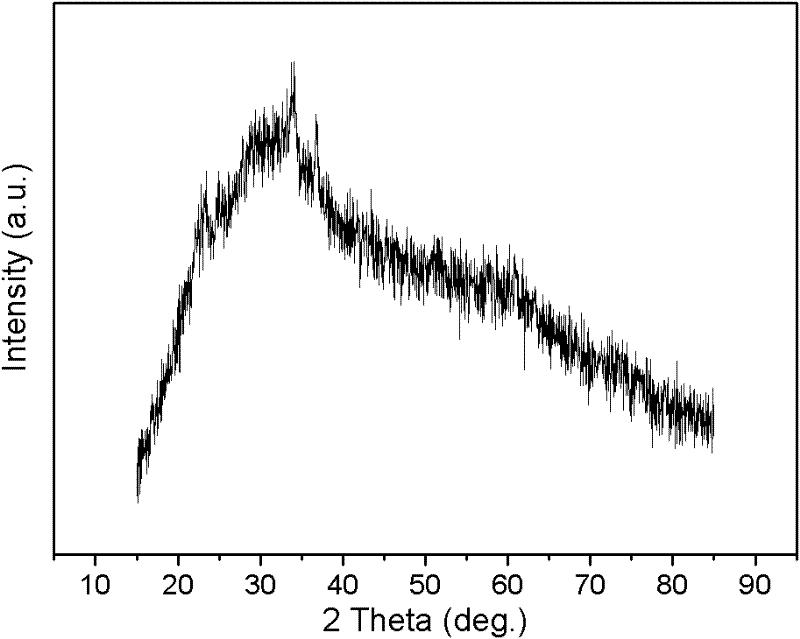
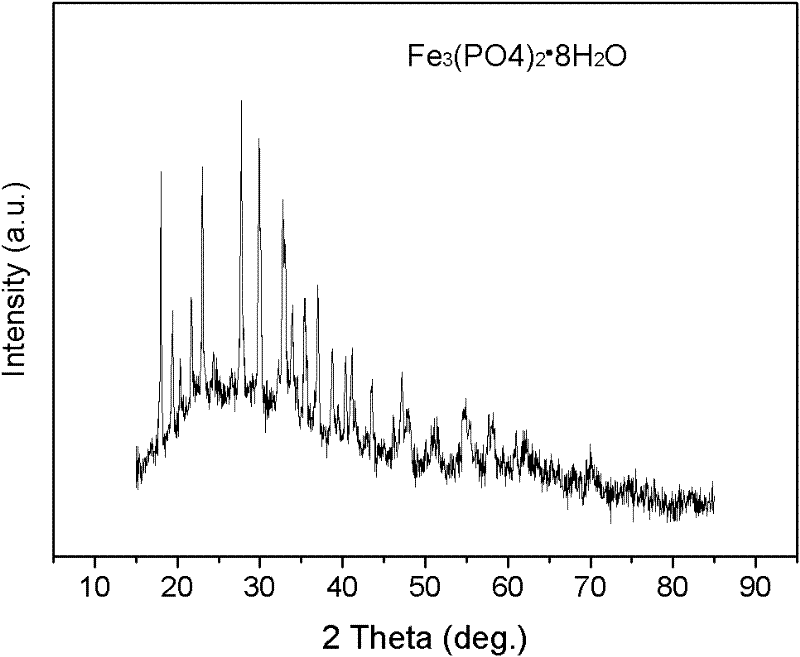
Method for preparing nanocrystal lithium iron phosphate anode material through co-precipitation
A technology for crystalline lithium iron phosphate and positive electrode materials, which is applied in the field of co-precipitation to prepare nanocrystalline lithium iron phosphate positive electrode materials, can solve the problems of amorphous ultrafine precursor powder, difficulty in obtaining, and inability to precipitate, and achieve excellent dispersion. The effect of flat discharge platform and uniform composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Mix an aqueous solution of a ferrous iron source compound, an aqueous solution of a lithium source compound, and an aqueous solution of a phosphorus source compound, and stir for 30 minutes at a stirring rate of 10,000 rpm to obtain a precursor solution. During the stirring process, the temperature of the mixture is controlled to be below 15° C.; The atomic percentages of iron, lithium and phosphorus in the precursor solution are 1:1:1, and the concentrations of the compounds in the aqueous solution of ferrous iron source compound, aqueous solution of lithium source compound and aqueous solution of phosphorus source compound are all 0.05mol / L, and the The divalent iron source compound is ferrous sulfate, the lithium source compound is lithium hydroxide, and the phosphorus source compound is dilithium hydrogen phosphate;
[0039] (2) The precursor solution described in step (1) was left to stand for 10 minutes, then washed with deionized water, filtered, and dried to ...
Embodiment 2
[0050] This embodiment is the same as Example 1, except that the divalent iron source compound is ferrous chloride or ferrous nitrate; the lithium source compound is lithium dihydrogen phosphate, dilithium hydrogen phosphate, lithium acetate , lithium nitrate, lithium sulfate or lithium chloride; the phosphorus source compound is lithium dihydrogen phosphate, ammonium phosphate, diammonium hydrogen phosphate, ammonium dihydrogen phosphate, sodium phosphate, disodium hydrogen phosphate, sodium dihydrogen phosphate, potassium phosphate , dipotassium hydrogen phosphate or potassium dihydrogen phosphate; the organic carbon source is sucrose, fructose, citric acid, ascorbic acid, cellulose or starch; the non-oxidizing atmosphere is nitrogen or hydrogen, or a mixed gas of nitrogen and hydrogen , or a mixed gas of argon and hydrogen; the inorganic carbon source is carbon black, carbon microspheres, carbon nanospheres, carbon nanotubes, carbon nanofibers or carbon gel; the binder is po...
Embodiment 3
[0053] (1) Mix the aqueous solution of the ferrous source compound, the aqueous solution of the lithium source compound and the aqueous solution of the phosphorus source compound, and stir for 120 minutes at a stirring rate of 3000 rpm to obtain a precursor solution. During the stirring process, the temperature of the mixture is controlled to be below 15° C.; The atomic percentages of iron, lithium and phosphorus in the precursor solution are 1:1.1:1, and the concentrations of the compounds in the aqueous solution of ferrous iron source compound, aqueous solution of lithium source compound and aqueous solution of phosphorus source compound are all 5 mol / L. The valence iron source compound is ferrous chloride, the lithium source compound is lithium acetate, and the phosphorus source compound is ammonium phosphate;
[0054] (2) The precursor solution described in step (1) was left to stand for 60 minutes, then washed with deionized water, filtered, and dried to obtain a pale yell...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com