Fusion protein of liver targeting peptide and human interferon a2b and its preparation method and application
A fusion protein and liver-targeting technology, applied in the biological field, can solve problems such as clearance, difficult to achieve precise cell targeting, and easy immune system of the body, and achieve the effects of improving specificity, precise and active targeted clearance, and reducing systemic dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Gene modification, fusion gene IFN-CSP recombination and construction of expression plasmid
[0036] In this example, Novagen's pET expression system was used to express the fusion gene IFN-CSP, the pET32a(+) plasmid was used as the expression vector, and the host cell was Escherichia coli.
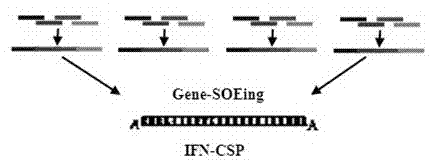
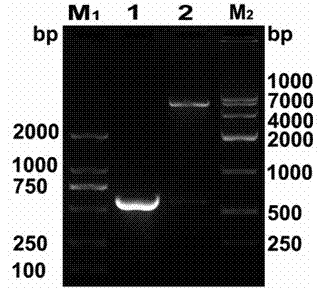
[0037] 1 Gene modification and fusion gene IFN-CSP recombination ( Figure 1-2 )
[0038] According to the amino acid sequence of human IFNa2b (NP_000596) and the amino acid sequence of Plasmodium falciparum CSP I-plus (CAA33421) published by NCBI, using E. coli preferred codons, computer-aided design of the nucleus of the liver-targeted interferon IFN-CSP gene Nucleotide sequence. The whole gene was divided into 16 overlapping oligonucleotide fragments, and primers P1-P16 were designed with Primer Premier 5.0 biological software. The primer sequences are shown in SEQ ID NO: 3-19. Where upstream P1 is introduced Kpn I restriction site, downstream P16 introduces a stop codon...
Embodiment 2
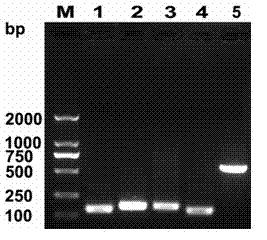
[0049] Example 2 Preparation of fusion protein IFN-CSP ( Figure 4 )
[0050] Transform the IFN-CSP / pET32a plasmid into the prepared expression host bacteria E.coli BL21 (DE3) competent cells, after ampicillin resistance screening, pick IFN-CSP / pET32a positive single colony into 5ml LB liquid medium (1wt% peptone, 0.5wt% yeast extract powder, 85 mmol / L Sodium chloride), 37℃, 220rpm shaking for 8~12h, and then this bacterial solution was inoculated into fresh LB liquid medium at a ratio of 1:100, 37℃, 220rpm shaking to OD600 about 0.6, add the final IPTG at a concentration of 1.0 mmol / L was induced for 4-6 hours. Collect the bacterial cells by centrifugation and add 1×SDS-PAGE Buffer (50mM Tris-HCl pH6.8, 100mM DTT, 2% SDS, 0.1wt% bromophenol blue, 10wt% glycerol), boil for 5min, centrifuge, and take the supernatant for sample. Make 6 vol% concentrated gel, 15 vol% separation gel, electrophoresis voltage: concentrated gel 80V, separation gel 120V. After the electrophoresis, t...
Embodiment 3
[0052] Example 3 In vitro pharmacodynamics and specific targeting effect on liver cells of the fusion protein IFN-CSP of the present invention
[0053] (1) In vitro pharmacodynamic study of IFN-CSP: HepG2.2.15 cells were used as the HBV infected cell model, and HepG 2.2.15 cells were cultured in culture medium containing different CSP-IFN concentration gradients, and the drug-containing culture was replaced every 3 days The supernatant was collected on the 3rd, 6th, and 9th day. HBsAg and HBeAg in the cell supernatant were detected by ELISA, and the A value was measured with a microplate reader, and the sample concentration was obtained by converting the A value of the standard product. The results show that CSP-IFN can significantly inhibit the secretion of HBsAg and HBeAg.
[0054] (2) The specific targeting effect of IFN-CSP on liver cells: paraffin-embedded mouse normal liver, kidney, heart, lung, and spleen tissues were taken respectively, and after routine deparaffinizatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com