Biodegradable Traditional Chinese Medicine Sustained-release Insert and Preparation for Ischemic Revascularization of Lower Limbs
A biodegradable and degradable technology, applied in the field of biodegradable traditional Chinese medicine slow-release implants and preparations for lower limb ischemic blood supply reconstruction, can solve the problems of lower limb ischemic diseases without puerarin or other traditional Chinese medicine, and achieve Improve blood perfusion of lower extremities, promote production, and be easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
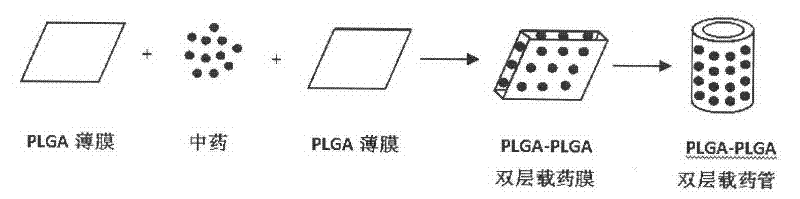
[0023] A method for preparing a biodegradable traditional Chinese medicine slow-release implant for ischemic revascularization of lower limbs comprises the following steps:
[0024] According to the ratio of 0.5g: 20ml, polylactic acid-polyglycolic acid copolymer (molar ratio 75 / 25, Mn10000, η0.43, Chengdu Organic Chemistry Co., Ltd., Chinese Academy of Sciences) was dissolved in dichloromethane (Tianjin Jiangtian Chemical Co., Ltd.) In the process, a biodegradable polymer solution was made, transferred to a tetrafluoroethylene mold, and a film was formed after the solvent evaporated completely, cut into strips, and 10 mg of puerarin (Puerarin, C 21 h 20 o 9 , 99.5%, Shanghai Merrill Chemical Technology Co., Ltd.) were uniformly dispersed between two films, fixed by solvent with methylene chloride, rolled into tubes with an inner diameter of 2mm and a wall thickness of 2mm, and cut into lengths of 10mm. A biodegradable traditional Chinese medicine slow-release insert for low...
Embodiment 2
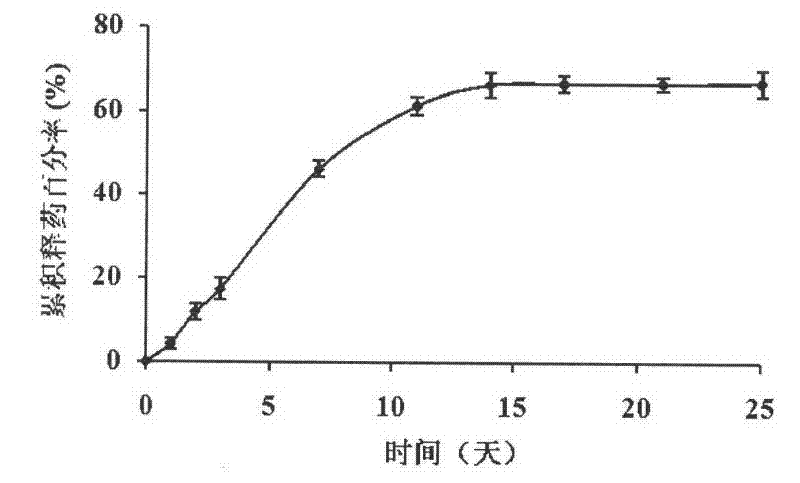
[0029] In vitro release test of inserts:
[0030]The content of puerarin was determined by high performance liquid chromatography. Chromatographic column: C18 column (200mm×4.6mm, 5μm, Kromasil); mobile phase: methanol-water (25:75); flow rate 1ml / min, detection wavelength 250nm, column temperature 30°C, injection volume 20μL. Under the above high-performance liquid chromatography conditions, the number of theoretical plates calculated according to the peak of puerarin is not less than 3000. Accurately weigh an appropriate amount of puerarin reference substance, add mobile phase to dissolve. And prepare 1.57, 3.14, 7.85, 15.7, 62.8, 94.2, 125.6, 157 μg / ml solutions, measure under the above-mentioned chromatographic conditions respectively, record the peak area. Regression analysis was carried out on the concentration by peak area, and the regression equation was obtained.
[0031] Put the insert into a sample tube containing 5ml of PBS buffer solution (pH7.4), and place it ...
Embodiment 3
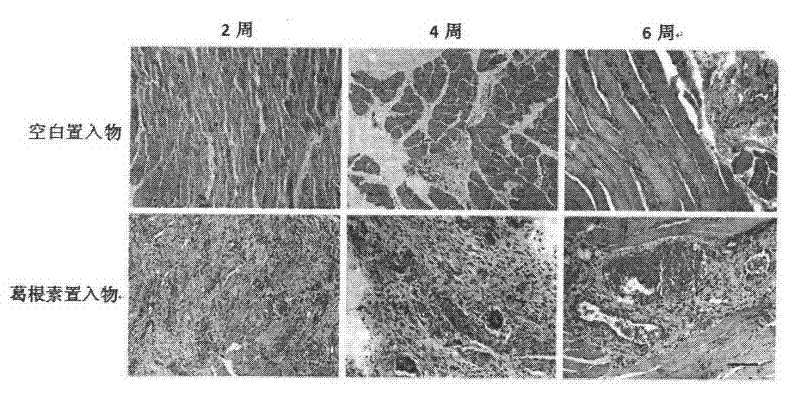
[0033] Animal experiment
[0034] (1) Establishment of acute lower limb ischemia model in rats
[0035] SPF-grade wistar rats, male, weighing 220-250 g, were provided by Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., and the license number is SCXK (Beijing) 2009-0004. Raising conditions: room temperature 23-25 degrees, rats were raised in stainless steel cages, free to drink and eat. Rats were fasted for 12 h before operation and had free access to water. Anesthetized by intraperitoneal injection of 10% chloral hydrate solution 0.3ml / 100g, fixed in supine position, routine skin preparation and disinfection of left lower extremity, longitudinal incision of the skin in the middle of left lower extremity, from groin to knee joint. The femoral artery was divided subgroin, ranging from the inguinal ligament to the knee joint, all branches of the femoral artery in the femur were ligated, and the femoral artery was cut under the inguinal ligament. After the oper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Wall thickness | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com