Method for preparing lithium iron phosphate-lithium vanadium phosphate by quenching method
A technology of lithium iron phosphate and lithium vanadium phosphate, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of unsatisfactory high-rate charge and discharge performance, poor low-temperature performance and rate performance, and unsatisfactory charge and discharge effect. Good high-rate charge-discharge performance, improved rate performance, and improved electronic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
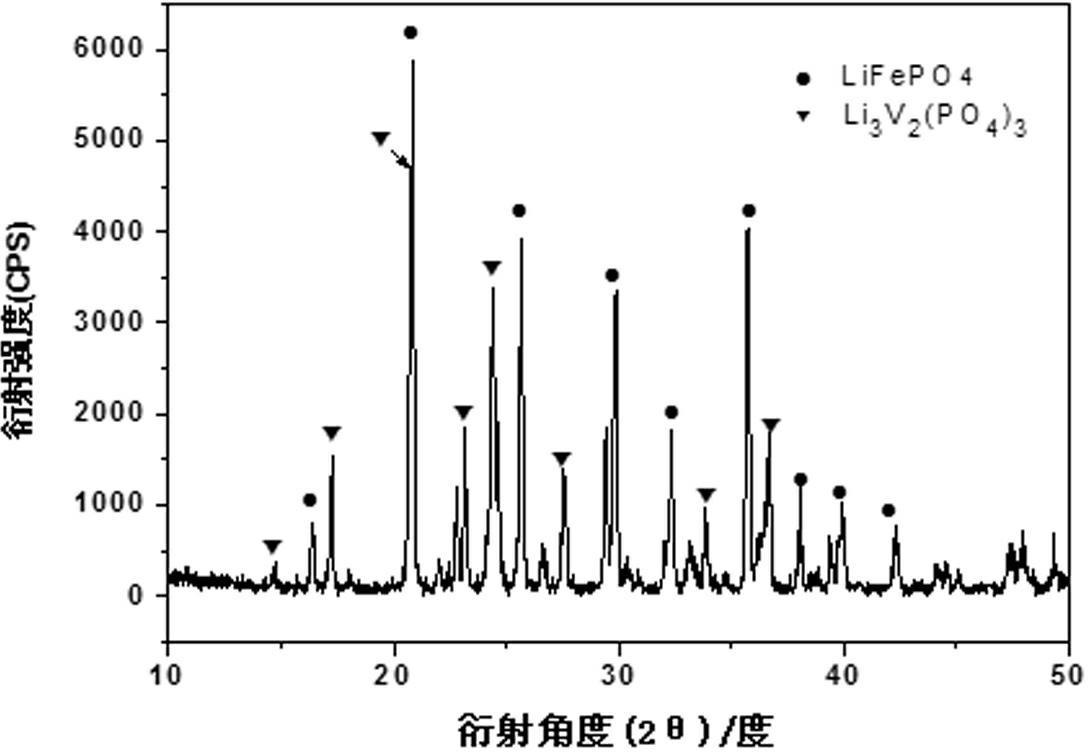
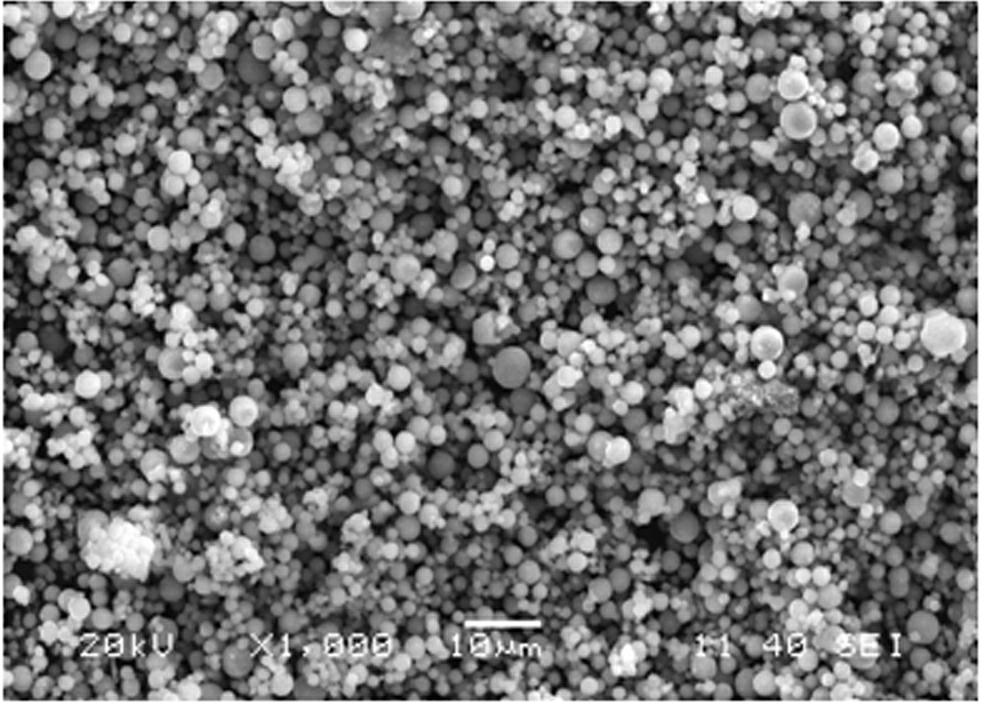
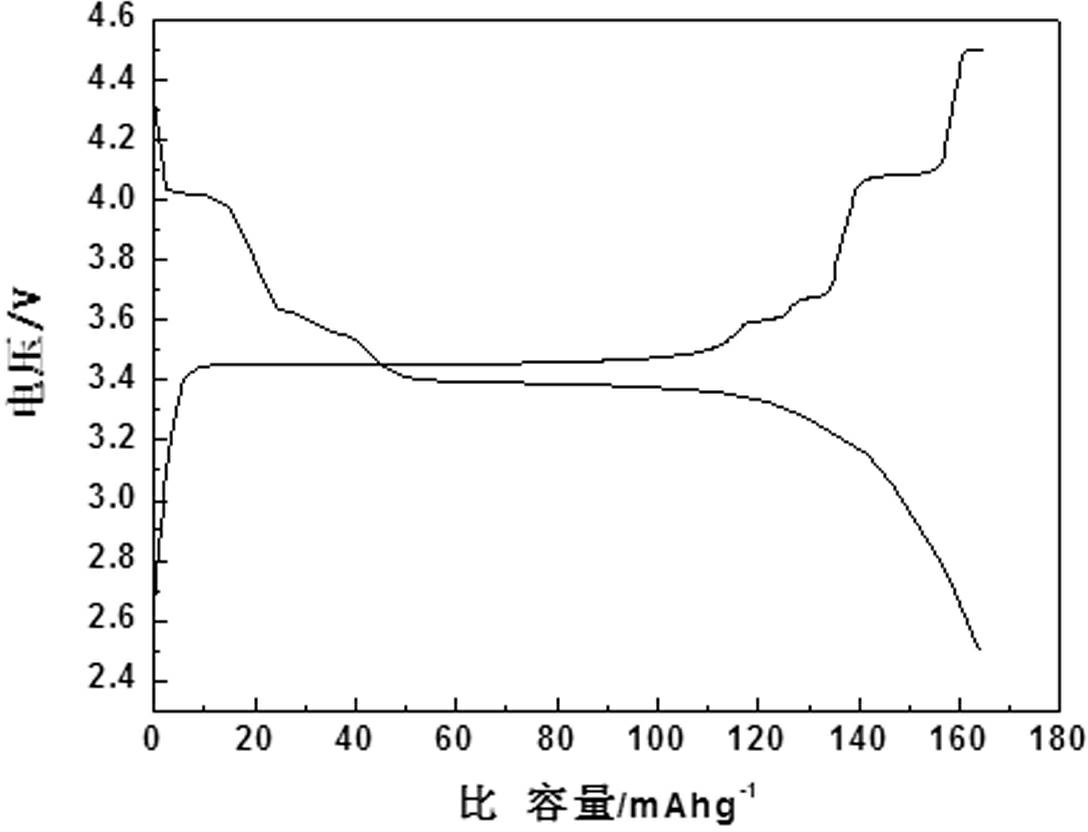
[0018] Using vanadium pentoxide, iron phosphate, lithium carbonate, and ammonium dihydrogen phosphate as raw materials, according to composite materials x LiFePO 4 · y Li 3 V 2 (PO 4 ) 3 The stoichiometric ratio of x : y =5:1 Mix evenly, add a certain amount of citric acid, stir and disperse evenly in water, then spray dry, then put it into a tube furnace, keep the temperature at 600°C for 18 hours under an argon atmosphere, and then put the high temperature The charge was quickly quenched in water at 25°C, liquid nitrogen at -193°C, and dry ice at -78°C for 0.5 hours, and the obtained material was analyzed by X-ray diffraction to be monoclinic and olivine. x LiFePO 4 · y Li 3 V 2 (PO 4 ) 3 composite structure. The product obtained by SEM is spherical, and the tap density is shown in Table 1. The resulting products were assembled into button batteries and charged and discharged at a rate of 10C. Their first discharge capacity is shown in Table 1
[0019] Table ...
Embodiment 2
[0022] Using vanadium dioxide, ferrous acetate, lithium formate, and triammonium phosphate as raw materials, according to composite materials x LiFePO 4 · y Li 3 V 2 (PO 4 ) 3 The stoichiometric ratio of x : y =10:1 mix evenly; add a certain amount of oxalic acid, stir and disperse evenly in ethanol, then spray dry, and then put it into a tube furnace, under a hydrogen atmosphere, keep the temperature at 700°C for 2 hours, and then put the high-temperature furnace charge Quickly placed in water at 0°C, 20°C, and 35°C for 1 hour, the obtained material was analyzed by X-ray diffraction as monoclinic crystal and olivine crystal, namely x LiFePO 4 · y Li 3 V 2 (PO 4 ) 3 composite structure. The product obtained by SEM is spherical, and the tap density is shown in Table 2. The resulting products were assembled into button batteries to charge and discharge at a rate of 10C, and their first discharge specific capacities are shown in Table 2
[0023] Table 2 Experiment...
Embodiment 3
[0026] Using ammonium metavanadate, ferrous oxalate, lithium oxide, diammonium hydrogen phosphate as raw materials, according to composite materials x LiFePO 4 · y Li 3 V 2 (PO 4 ) 3 The stoichiometric ratio of x : y =1:10 mixed evenly; add a certain amount of sucrose, stir and disperse evenly in acetone, then spray dry, and then put it into a tube furnace, under a nitrogen atmosphere, keep the temperature at 800°C for 15 hours, Quickly placed in dry ice at -78.5°C for 5 minutes, the obtained material was analyzed by X-ray diffraction as monoclinic and olivine, namely x LiFePO 4 · y Li 3 V 2 (PO 4 ) 3 composite structure. The spherical shape of the product can be obtained by SEM, and the tap density is as high as 1.50 g cm -3 . The obtained product was assembled into a button battery and charged and discharged at a rate of 10C, and the specific capacity of the first discharge was 138mAh·g -1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tap density | aaaaa | aaaaa |
| Tap density | aaaaa | aaaaa |
| Tap density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com