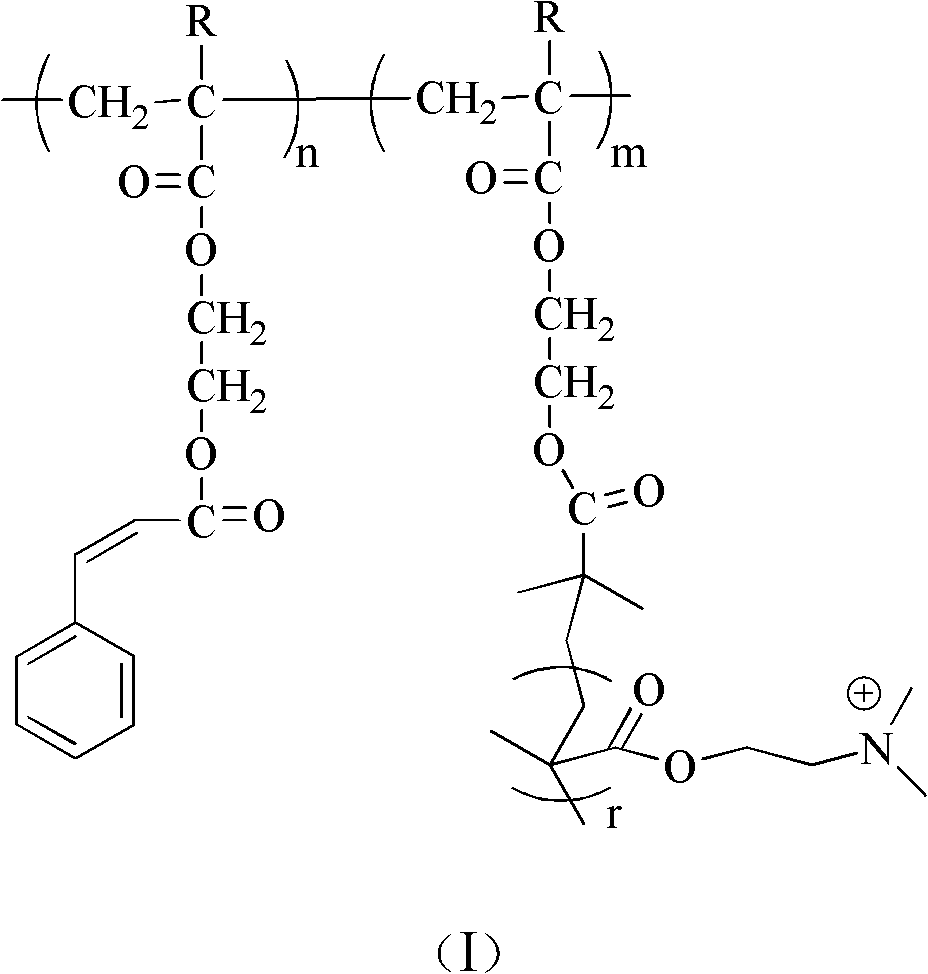
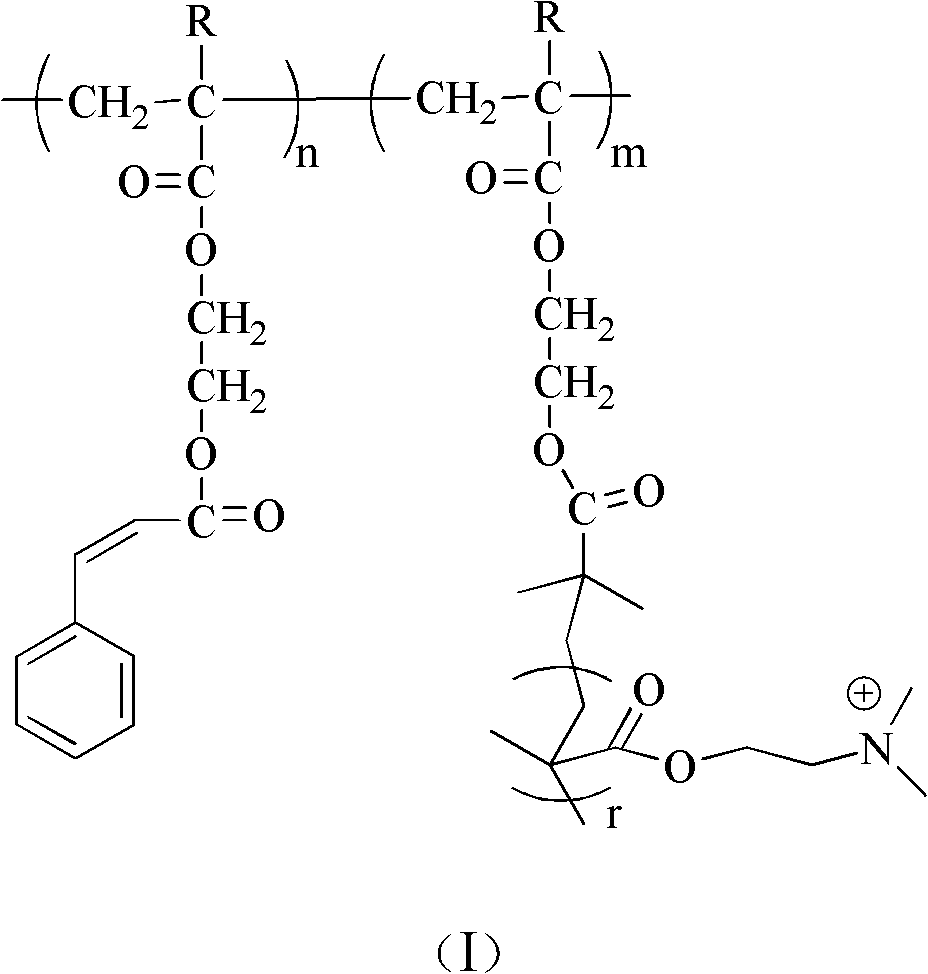
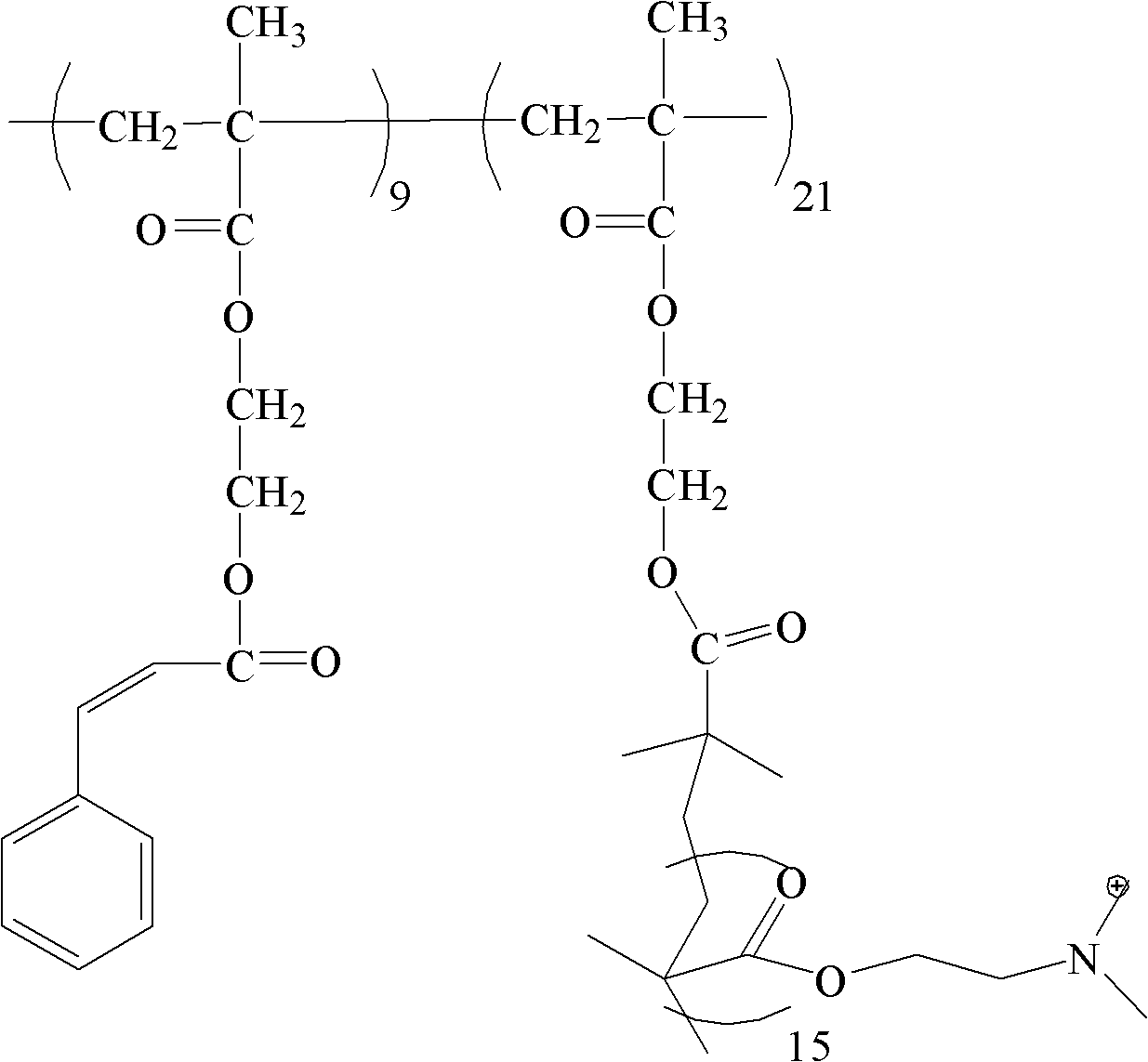
Poly(2-hydroxyethyl methacrylate) and anion exchange membrane for vanadium battery
A polyhydroxyethyl methacrylate and anion exchange membrane technology, applied in the field of electrochemical materials, can solve the problems of complex process, high production cost and high surface resistance, and achieve easy, environment-friendly, low cost and good vanadium resistance performance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A synthetic method for polyhydroxyethyl methacrylate, comprising the following steps:
[0049] (1) In the round bottom flask, add 1mmol 4-bromomethylbenzophenone, 50mmol hydroxyethyl methacrylate, 1mmol cuprous bromide, 1mmol pentamethyldiethylenetriamine and 6.0g toluene solution, to react The system was subjected to liquid nitrogen freezing-vacuumizing-argon filling-thawing repeated operation 3 times, and the system was sealed after 10 minutes of argon gas. The reaction was carried out at 20°C for 5 hours. Terminate the reaction, centrifuge the reaction product, take the precipitate, and wash the precipitate to obtain polytrimethyl-terminated hydroxyethyl methacrylate (PHEMA-tmsn); wherein n is 30.
[0050] (2) Dissolve 1mmol PHEMA-tmsn in 10ml tetrahydrofuran, add 0.1ml 1mol / L hydrochloric acid solution for hydrolysis, react at 30°C for 3 hours, centrifuge the reaction product, take the precipitate, wash the precipitate, and obtain polyhydroxyethyl methacrylate (PH...
Embodiment 2
[0060] A synthetic method for polyhydroxyethyl methacrylate, comprising the following steps:
[0061] (1) In the round bottom flask, add 1mmol 1-phenylethyl chloride, 100mmol hydroxyethyl acrylate, 3mmol cuprous bromide, 3mmol pentamethyldiethylenetriamine and 11.6g anisole solution, and carry out the reaction system Liquid nitrogen freezing-vacuumizing-filling with argon-thawing operation repeated 3 times, argon for 10 minutes and then sealed. The reaction was carried out at 50° C. for 8 hours. Terminate the reaction, centrifuge the reaction product, take the precipitate, and wash the precipitate to obtain polytrimethyl-terminated hydroxyethyl acrylate (PHEA-tmsn); wherein n is 70.
[0062] (2) Dissolve 1mmol PHEA-tmsn in 10ml dioxane, add 0.1ml 1mol / L hydrochloric acid solution for hydrolysis, react at 30°C for 6 hours, centrifuge the reaction product, take the precipitate, wash the precipitate, and obtain polyhydroxyethyl acrylate Esters (PHEAn).
[0063] (3) Dissolve 1m...
Embodiment 3
[0072] A synthetic method for polyhydroxyethyl methacrylate, comprising the following steps:
[0073] (1) In the round bottom flask, add 1mmol 1-phenylethyl chloride, 400mmol hydroxyethyl methacrylate, 8mmol cuprous chloride, 8mmol 2'2-bipyridyl and 52.1g anisole solution, and the reaction system Perform liquid nitrogen freezing-vacuumizing-filling with argon-thawing repeatedly 3 times, and seal it after argon gas for 10 minutes. The reaction was carried out at 80° C. for 15 hours. Terminate the reaction, centrifuge the reaction product, take the precipitate, and wash the precipitate to obtain polytrimethyl-terminated hydroxyethyl acrylate (PHEMA-tmsn); wherein n is 280.
[0074] (2) Dissolve 1mmol PHEMA-tmsn in 10ml pyridine, add 0.1ml 1mol / L hydrochloric acid solution for hydrolysis, react at 30°C for 9 hours, centrifuge the reaction product, take the precipitate, wash the precipitate to obtain polyhydroxyethyl methacrylate (PHEMAn).
[0075] (3) Dissolve 1mmol PHEMAn in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com