Oxazole thiadiazole organic copper compound, its preparation method, preparation and application in controlling agricultural plant diseases
A technology for oxazole thiadiazoles and oxazole thiadiazoles is applied in the field of preventing and controlling agricultural plant diseases, oxazole thiadiazole type organo-copper compounds and their preparation fields, and can solve the phytotoxicity of sensitive plants, the value-added of rust tick, Crop phytotoxicity and other problems, to achieve the effects of easy industrial production, mild reaction conditions, and broad bactericidal spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
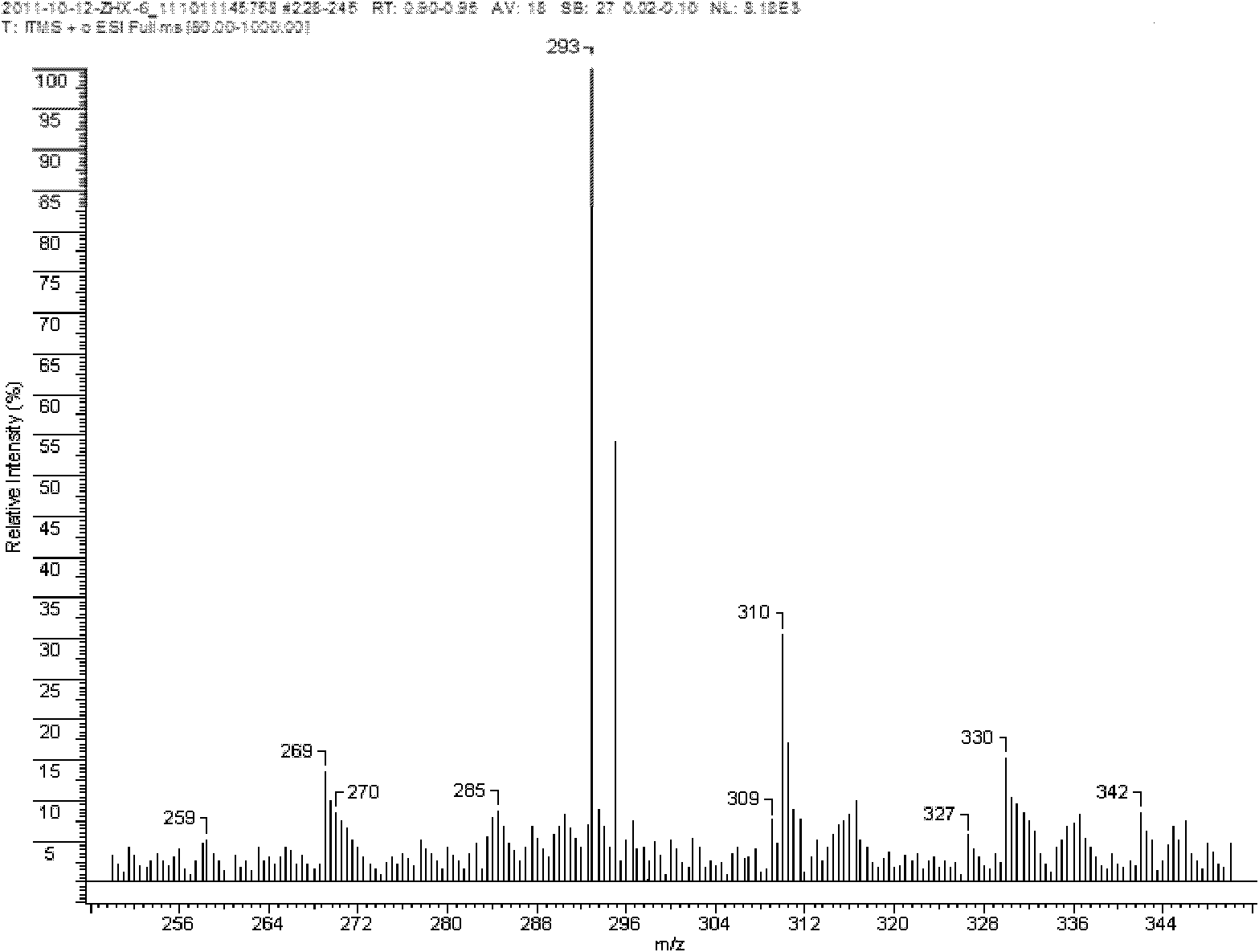
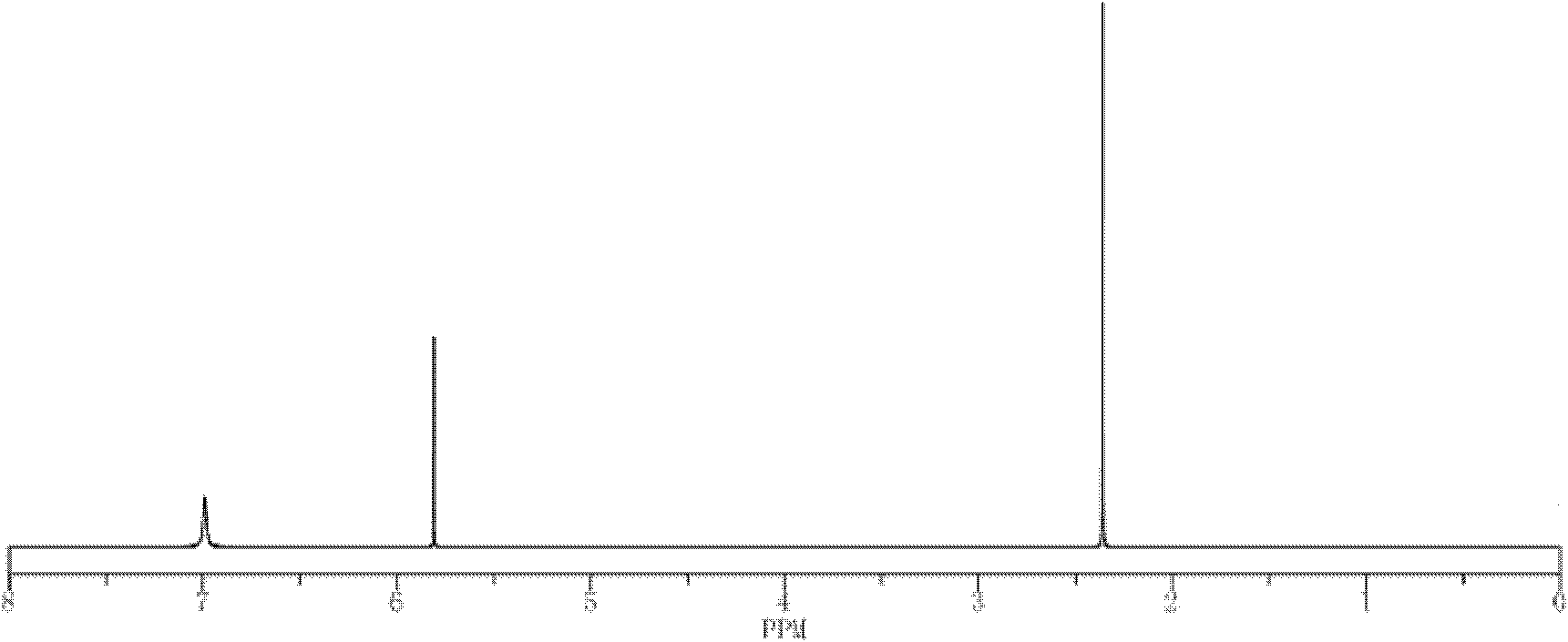
[0029] 6.6g of 99% purity of 3-hydroxyl-5-methylisoxazole, 5.4g of 98% of 2-amino-5-mercapto-1,3,4-thiadiazole and 160ml of 75% mass fraction The ethanol aqueous solution of % is thrown into 250ml reaction bottle at one time, is heated and dissolved at the temperature of 72 ℃, obtains solution A, and 2.5g purity is 99% copper sulfate pentahydrate and 25ml distilled water to join in the 250ml reaction bottle, heat, and Stir at a temperature of 44°C to make it fully dissolve. After the copper sulfate is completely dissolved in water, under the action of stirring, add solution A dropwise (0.02-0.05ml per drop) into the aqueous solution of copper sulfate. The dropwise addition is completed within 28 minutes, then the temperature is controlled at 70°C, and the reaction is stirred for 1 hour. After the reaction, the precipitation is allowed to stand for 3.5 hours. Washed with water, and then dried in a constant temperature drying oven at 105 ° C to obtain a yellow-green powder that ...
Embodiment 2
[0035] The preparation method of copper-3-hydroxyl-5-methylisoxazole-2-amino-5-mercapto-1,3,4-thiadiazole is the same as in Example 1.
[0036] Take the following materials in percentage by weight: 20.0% of copper-3-hydroxyl-5-methylisoxazole-2-amino-5-mercapto-1,3,4-thiadiazole prepared in this example, 1.0% octylphenol polyoxyethylene ether, 4.5% dibutyl naphthalene sulfonate sodium formaldehyde condensate, 74.5% white carbon black, according to the above ratio, mix the materials thoroughly, grind and pass through a 320-mesh sieve to obtain 20% Wettable powder of oxazole thiadiazole organocopper compound.
[0037] The tested strain is bacterial blight of rice, which is provided by the Pesticide Research Institute of Northwest Agriculture and Forestry University. The prepared 20% oxazolethiadiazole organic copper compound wettable powder is determined by routine antibacterial test according to the conventional method. 20% wettable powder of hymexazol or carbendazim (the exci...
Embodiment 3
[0039] 6.5g of 3-hydroxyl-5-methylisoxazole with 99% purity, 5.6g of 98% 2-amino-5-mercapto-1,3,4-thiadiazole and 240ml with a mass fraction of 95 The methanol aqueous solution of % is thrown into 500ml reaction bottle at one time, is heated and dissolved at the temperature of 85 ℃, obtains solution A, is that 99% copper sulfate pentahydrate and 25ml distilled water are added in the 500ml reaction bottle with 2.5g purity, heat, and Stir at a temperature of 35°C to make it fully dissolve. After the copper sulfate is completely dissolved in water, under the action of stirring, add solution A dropwise (0.02-0.05ml per drop) into the aqueous solution of copper sulfate. The dropwise addition is completed within 25 minutes, then the temperature is controlled at 65°C, and the reaction is stirred for 1 hour. After the reaction, the precipitation is allowed to stand for 4 hours. Wash, and then dry in a constant temperature drying oven at 110 ° C to obtain a yellow-green powder that is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com