Antigen conformation epitope mimic peptide of hepatitis C virus F protein and application thereof
A hepatitis C virus and conformational epitope technology, applied in the field of biomedicine, can solve the problems of high preparation cost, difficulty in realization, and complexity, and achieve the effects of stable phage particles, no need for purification process, and good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
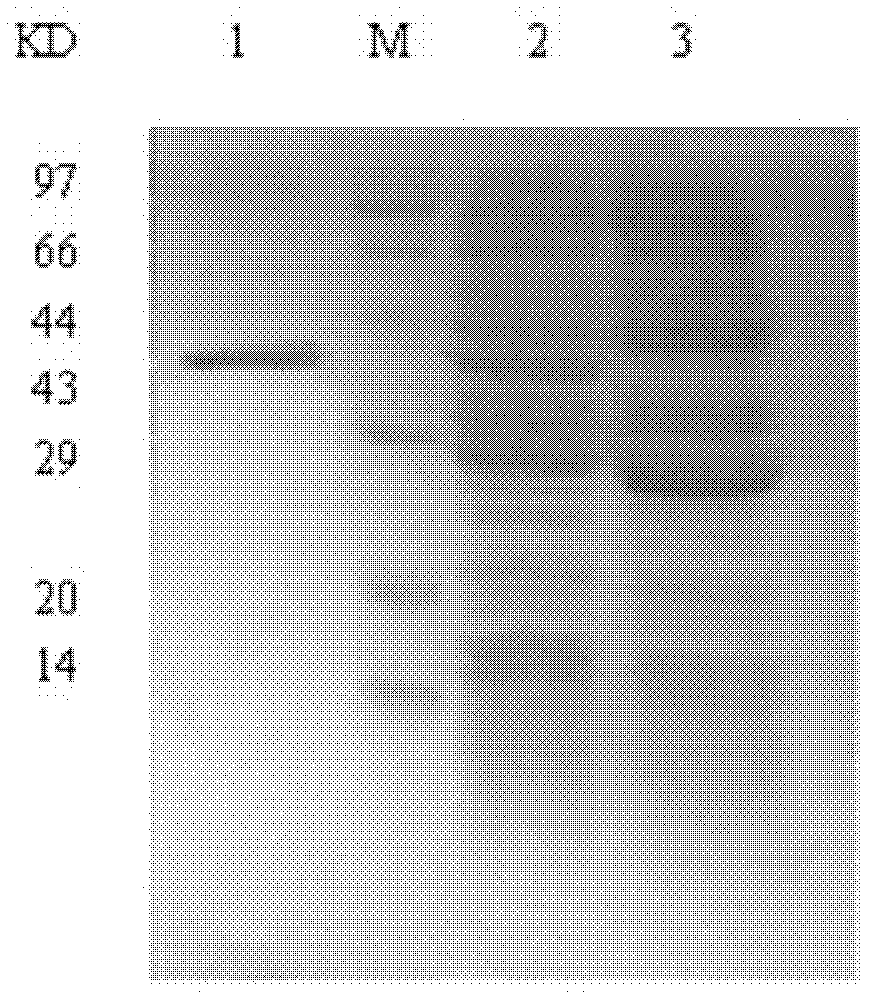
[0037] Embodiment 1, prokaryotic expression and purification of hepatitis C virus F protein
[0038] 1. Materials: E.coli TG1 was purchased from Stratagen. IPTG was purchased from Promega Corporation. Glutathione Sepharose 4B gel was purchased from Pharmacia. The PCR kit was purchased from Shenergy Gaming Company. Primers were synthesized by Shanghai Boya Company. Various restriction endonucleases, DNA ligases, vectors and protein markers were purchased from TaKaRa Company. DNA gel recovery kit was purchased from Shanghai Huashun Company.
[0039] 2. Method:
[0040] 1) Primers
[0041] Upstream primer P1: 5′-GT AGCACAAATCCTAAGCCTCAGAG-3' (SEQ ID NO. 2);
[0042] Upstream primer P2: 5'-CTAAGCCTCAGAGAAAGCCAAACGTAACACC-3' (SEQ ID NO.3);
[0043] Upstream primer P3: 5′-GT CCAAACGTAACACC-3' (SEQ ID NO. 4);
[0044] Downstream primer P4: 5′-GA GCAACCAGGCAGA-3' (SEQ ID NO. 5).
[0045] The above primers refer to the master thesis "Cloning, Expression and Preliminary ...
Embodiment 2
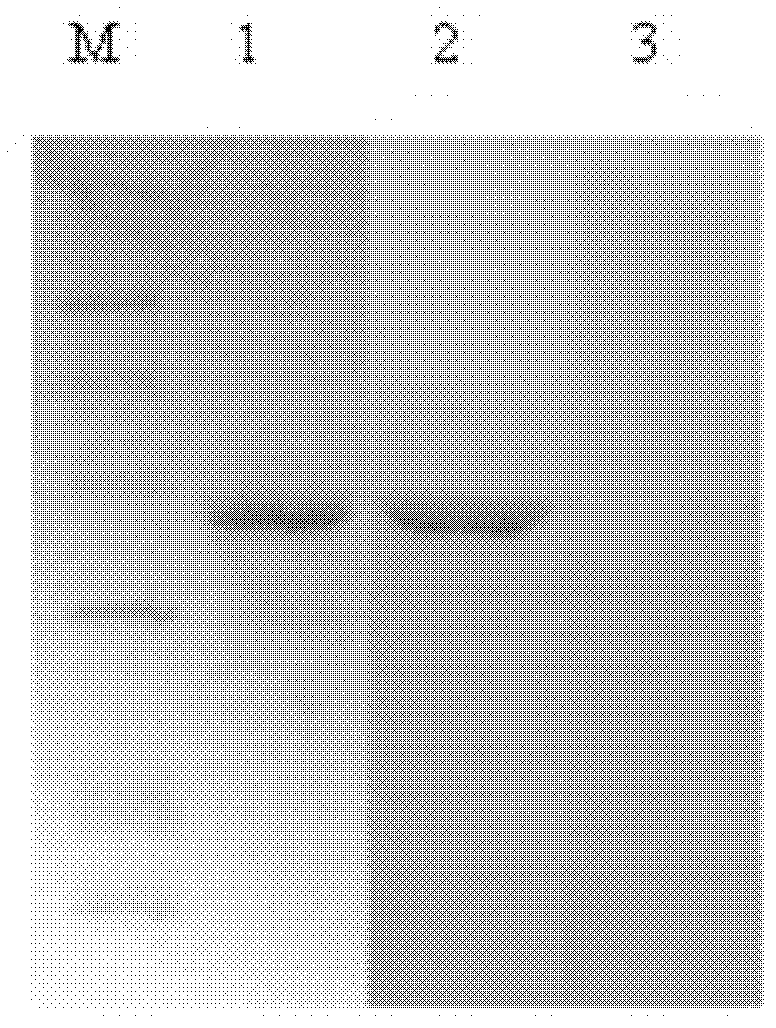
[0055] Example 2, Purification and Identification of F Protein Antibody
[0056] 1) Materials: HRP-labeled goat anti-mouse IgG was purchased from Nanjing Arendi Company. BALB / c mice aged 6-8 weeks were purchased from Shanghai Slack Experimental Animal Co., Ltd. and raised by the Military Medical Research Institute of Nanjing Military Region. Freund's complete adjuvant and Freund's incomplete adjuvant are both sigma products. Western blot kit was purchased from Huamei Bioengineering Company.
[0057] 2) Preparation of anti-HCV-F antibody The concentration of purified HCV-F measured by spectrophotometer is 1 μg / μL, and the antigen and adjuvant are mixed according to the volume ratio of 1:1, and repeatedly sucked with a sterile syringe for 30 minutes to make it completely emulsified . Two mice were used as blank controls, and eight mice were used for immune experiments. In the first week, the mice were subcutaneously injected with the emulsified antigen and Freund's complete ad...
Embodiment 3
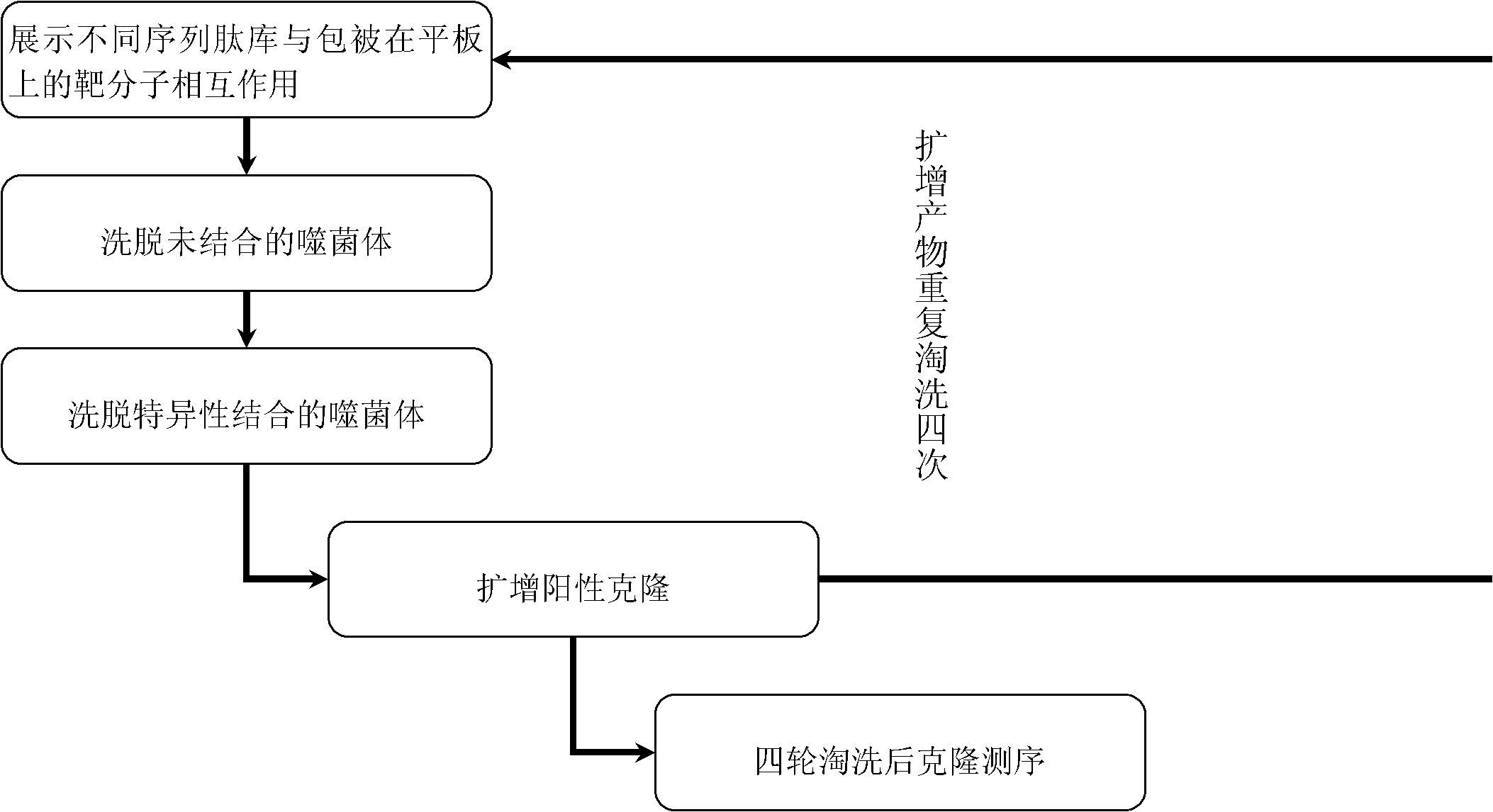
[0060] Embodiment 3, phage random peptide library screening
[0061] 1) M13 phage dodecapeptide library (Ph.D.-12 TM Phage Display Peptide Library Kit) purchased from New England Biolabs company
[0062] 2) Screening method: Coat the microtiter plate with mouse anti-HCV-F antibody (100 μg / ml) diluted in coating solution (carbonic acid buffer solution, pH 9.6), overnight at 4°C, add blocking solution at 4°C for 2-3 hours, wash with TBST, and spin dry the washing solution. Add diluted phage random peptide library to each well, and the number of phage added in each round should be 2×10 10-11 CFU, incubate at room temperature for 1 hour, shake off the liquid, rinse with TBST buffer containing 0.1% Tween-20 (v / v), and finally use 100 μl pH 2.2 glycine-hydrochloric acid buffer to elute the protein specifically bound to the F protein antibody phage, and immediately neutralized with 10 μl pH 9.8 Tris buffer, 10 μl was taken for phage titer determination, and the remaining phages w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com