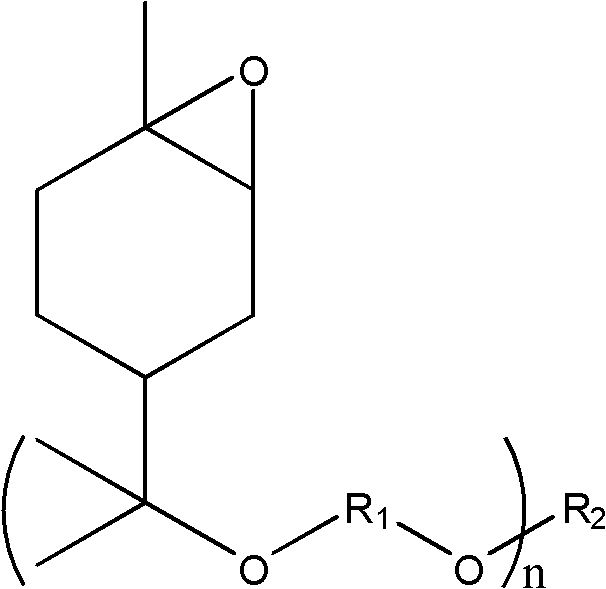
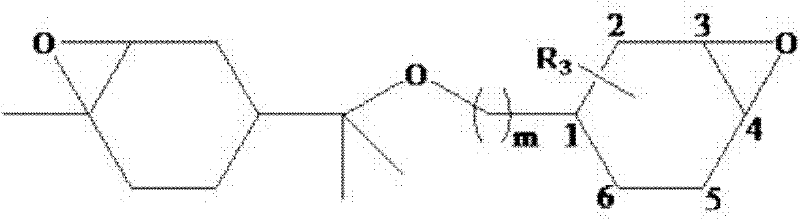
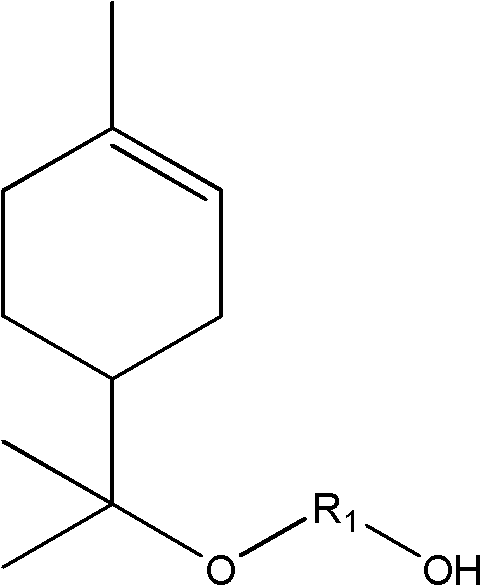
Cycloaliphatic epoxy compound and preparation method and application thereof
A cycloaliphatic epoxy and compound technology, applied in the field of compound preparation, can solve the problems of poor mechanical properties and processing properties, lack of chemical resistance, microcircuit corrosion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] When reaction starting raw material is limonene and aliphatic glycol, the preparation method of cycloaliphatic epoxy compound is as follows:
[0080] (1) 10mmol limonene, 11mmol 1,4-butanediol and 2.0mmol iron trichloride were added to 45mL chloroform solvent cooled in an ice bath, heated to 25°C after magnetic stirring for 60 minutes, passed through a developing agent (by Petroleum ether: ethyl acetate is composed of 3:1 by volume) TLC monitors the reaction process until the raw material disappears on the TLC thin-layer plate or no longer decreases, adding water to terminate the reaction, extracting the aqueous layer with dichloromethane, combining the organic layers, and reducing The solvent was distilled off under pressure; the product was purified by column chromatography (eluent: petroleum ether: ethyl acetate in a volume ratio of 3:1).
[0081] The resulting product is detected by nuclear magnetic resonance, and the obtained data are as follows: 1 HNMR (400MHz, C...
Embodiment 2
[0094] When reaction starting raw material is limonene and aliphatic glycol, the preparation method of cycloaliphatic epoxy compound is as follows:
[0095] (1) 10mmol limonene, 13mmol 1,3-propanediol and 2.5mmol ferric trichloride are added in the 50mL acetonitrile solvent that the ice bath cools down, and the gained mixed solution is magnetically stirred for 30 minutes and then warmed up to 35°C, passed through the developing agent (by Petroleum ether: ethyl acetate by volume ratio 3: 1 composition) TLC monitors the reaction process. When the raw material disappears on the TLC thin-layer plate or no longer decreases, water is added to terminate the reaction. The aqueous layer is extracted with dichloromethane, and the organic layer is combined. The solvent was distilled off; the product was purified by column chromatography (eluent: petroleum ether: ethyl acetate at a volume ratio of 5:1).
[0096] The resulting product is detected by nuclear magnetic resonance, and the obta...
Embodiment 3
[0109] When reaction starting raw material is limonene and cycloaliphatic olefin monohydric alcohol, the preparation method of cycloaliphatic epoxy compound is as follows:
[0110] (1) 10mmol limonene, 10mmol cyclohexene alkyl alcohol and 2.0mmol anhydrous ferric trichloride were added to 45mL methylene chloride solvent cooled in an ice bath, and the temperature was raised to 45°C after magnetic stirring for 40 minutes, and the developer ( Composed of petroleum ether: ethyl acetate in a volume ratio of 4:1) TLC monitors the reaction process until the raw material disappears on the TLC thin layer plate or no longer decreases, adding water to terminate the reaction, extracting the water layer with dichloromethane, combining the organic layers, and The residual solvent in the organic layer was distilled off under reduced pressure, and the obtained crude product was purified with a column chromatography eluent (petroleum ether: ethyl acetate in a volume ratio of 3:1);
[0111] The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com