Preparation method and purpose for iron oxide-based anode material for lithium ion battery
A technology for ion batteries and negative electrode materials, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of cycle performance degradation, limited practical application, low conductivity, etc., and achieve improved cycle stability, good electrochemical performance, cycle The effect of excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The preparation method of iron oxide-based lithium ion battery negative electrode material comprises the following steps:
[0021] 1) Dissolve the iron salt precursor in water, mix well and then add combustion agent, the molar ratio of combustion agent to iron ion is 0.5:1-4:1, mix well and then add ammonia water to adjust the pH of the solution to 6.5-7.5 ;
[0022] 2) Heat and evaporate the solution in a water bath, keep the temperature constant at 70-100°C, and stir continuously for 1-4 hours until a viscous gel is formed;
[0023] 3) Put the gel in a muffle furnace pre-heated to 200-500°C for combustion reaction, and keep it warm for 1 hour after the combustion reaction to obtain Fe 2 o 3 Powder;
[0024] 4) Fe 2 o 3 The powder and the organic carbon source are ground and mixed at a mass ratio of 1:0.01 to 1:1, then placed in a tube furnace, and then heat treated at 400 to 800°C for 1 to 10 hours in an argon atmosphere to obtain Fe. 3 o 4 / C composites, where...
Embodiment 1
[0028] 1) Dissolve ferric nitrate in water, mix well and then add citric acid, the molar ratio of citric acid to iron ion is 2:1, mix well and then add ammonia water to adjust the pH of the solution to 7;
[0029] 2) Heat and evaporate the solution in a water bath, keep the temperature constant at 80°C, and stir continuously for 1 to 4 hours until a viscous gel is formed;
[0030] 3) Put the gel in a muffle furnace preheated to 500°C for combustion reaction, and keep it warm for 1 hour after the combustion reaction to obtain fluffy Fe 2 o 3 Powder;
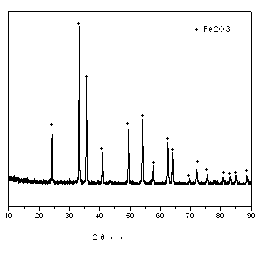
[0031] From figure 1 The Fe prepared in Example 1 2 o 3 The X-ray diffraction pattern of the powder shows that the position of the diffraction peak of the sample is the same as that of Fe in the standard spectrum. 2 o 3 (JCPDS No33-0664) The diffraction peak positions are consistent, proving that the prepared Fe is pure 2 o 3 Mutually.
[0032] figure 2 is the Fe prepared in Example 1 2 o 3 The scanning electron micr...
Embodiment 2
[0037] 1) Dissolve ferric nitrate in water, mix well and then add citric acid, the molar ratio of citric acid to iron ion is 2:1, mix well and then add ammonia water to adjust the pH of the solution to 7;
[0038] 2) Heat and evaporate the solution in a water bath, keep the temperature constant at 80°C, and stir continuously for 1 to 4 hours until a viscous gel is formed;
[0039] 3) Put the gel in a muffle furnace preheated to 500°C for combustion reaction, and keep it warm for 1 hour after the combustion reaction to obtain fluffy Fe 2 o 3 Powder;
[0040] 4) Fe 2 o 3 The powder and sucrose are ground and mixed at a mass ratio of 1:0.6, then placed in a tube furnace, and heat-treated at 500°C for 2 hours in an argon atmosphere to incompletely burn the sucrose and reduce Fe 2 o 3 , get Fe 3 o 4 / C composites, where carbon accounts for Fe 3 o 4 The mass fraction of / C is 13.34%.
[0041] From Figure 4 The Fe prepared in Example 2 3 o 4 The X-ray diffraction patter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com