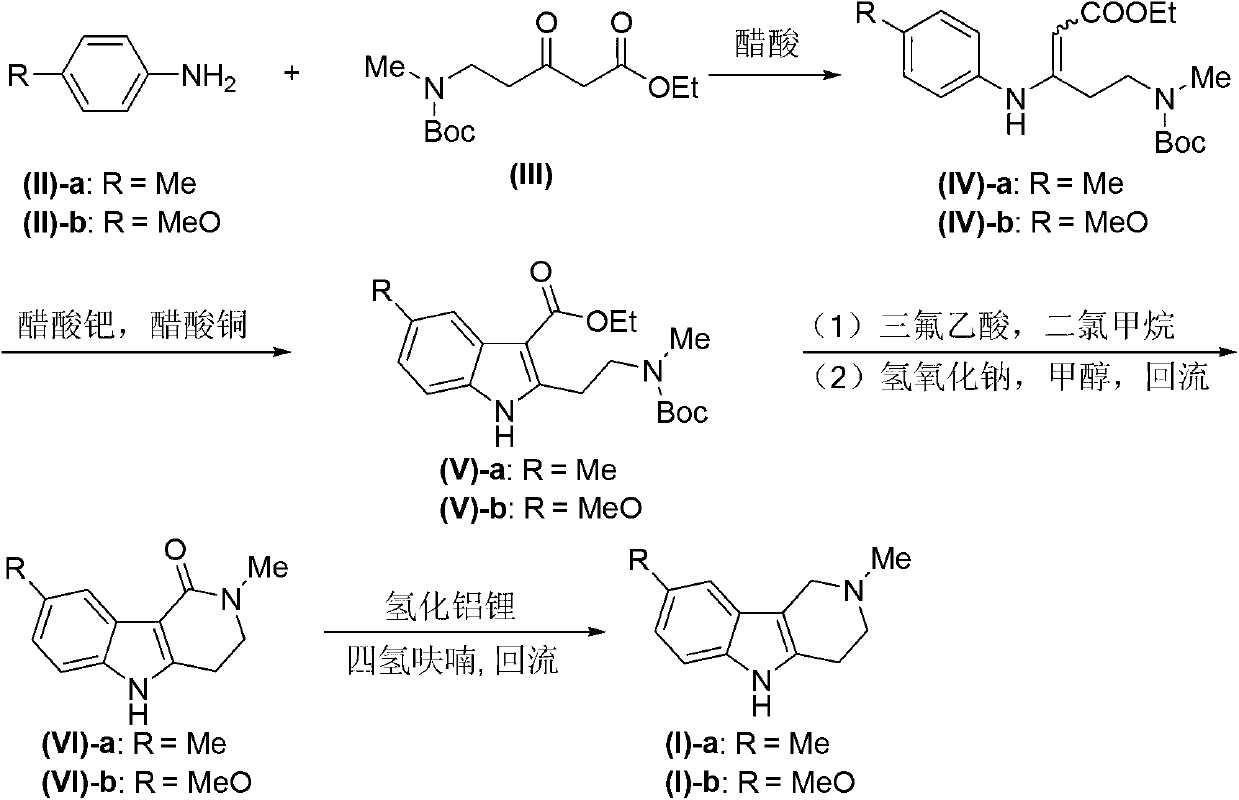
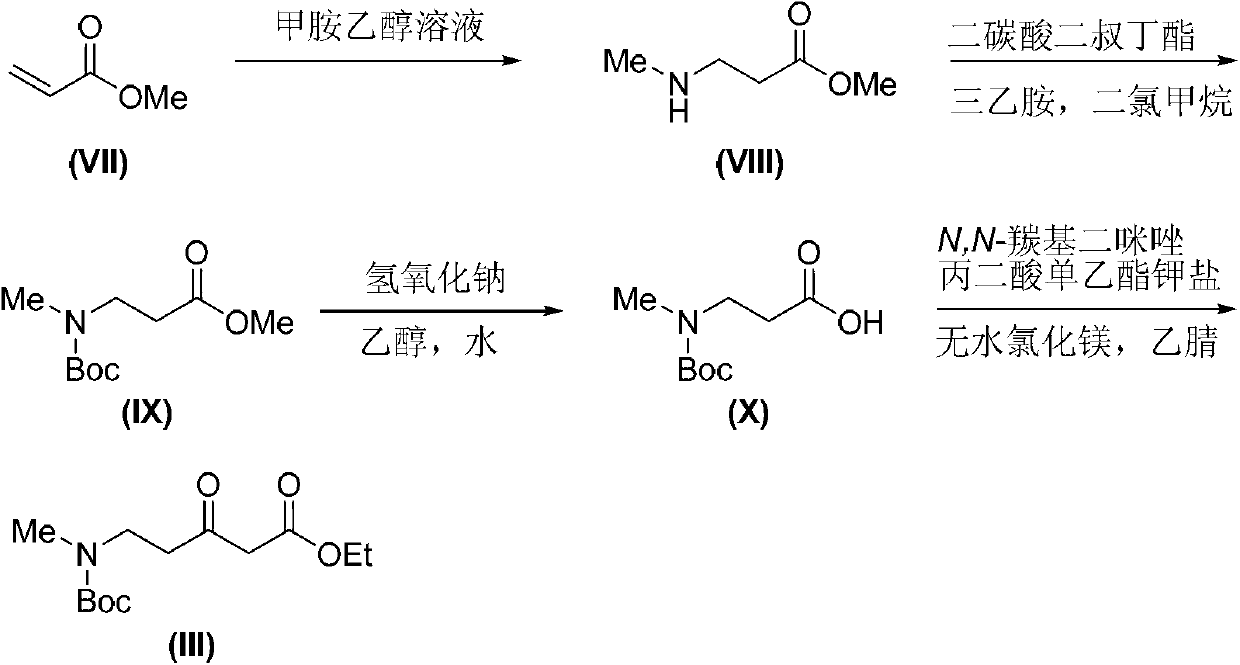
Tetrahydro-gamma-carboline derivative synthesis method
A synthetic method and derivative technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of limited application range, indispensable ortho-fluorine substituents, etc., and achieve the effect of simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of 3-(methylamino)methyl propionate (VIII)
[0043] Methylamine ethanol solution (33% methylamine mass fraction, 320mmol) was placed in a 100mL round bottom flask, methyl acrylate (VII) (6.9g, 80mmol) was dissolved in ethanol (15mL) and placed in a constant pressure dropping funnel Slowly add the ethanol solution of methyl acrylate (VII) dropwise to the methylamine ethanol solution at minus 20°C. After the dropwise addition, continue the reaction at minus 20°C for 8 hours, return to normal temperature after the reaction, and remove the solvent under reduced pressure and unreacted methylamine, and the residue was separated and purified by vacuum distillation to obtain 8.0 g of a colorless oily liquid, yield: 86%.
[0044] Experiments have proved that when the reaction temperature is selected at minus 10°C, the preparation of methyl 3-(methylamino)propionate (VIII) can also be completed in the same manner as in this example.
Embodiment 2
[0046] Preparation of 3-[(tert-butoxycarbonyl)(methyl)amino]propionic acid methyl ester (IX)
[0047] Methyl 3-(methylamino)propionate (VIII) (9.4 g, 80 mmol) was dissolved in dichloromethane (200 mL), and triethylamine (22 mL, 160 mmol) and di-tert-butyl dicarbonate were added to the solution (19g, 88mmol). Stir the reaction at room temperature for 5 hours, and when the reaction is complete, add a saturated solution of ammonium chloride (100 mL) to the reaction solution and stir for half an hour, collect the organic layer in a separatory funnel, wash with water (150 mL), and wash the organic phase with anhydrous After drying over sodium sulfate and concentrating under reduced pressure, a crude product was obtained. The crude product was separated and purified by column chromatography to obtain 17.4 g of a colorless oily liquid, yield: 94%.
[0048] 1 H NMR (400MHz, CDCl 3 ): δ3.69(s,3H),3.51(t,J=6.8Hz,2H),2.87(s,3H),2.55(t,J=6.8Hz,2H),1.46(s,9H). 13C NMR (101MHz, CDCl 3 ...
Embodiment 3
[0050] Preparation of 3-[(tert-butoxycarbonyl)(methyl)amino]propionic acid (X)
[0051] 3-[(tert-butoxycarbonyl)(methyl)amino]propionic acid methyl ester (IX) (6.5g, 30mmol) was dissolved in ethanol (150mL), and an aqueous solution of sodium hydroxide (1.8 g, 45mmol, 50mL), stirred and reacted at room temperature for 6 hours, removed ethanol under reduced pressure, adjusted the pH of the solution to 3 with hydrochloric acid (2N), placed the resulting aqueous solution in a separatory funnel, extracted with ethyl acetate (150mL), collected And the organic phase was concentrated to obtain 5.8 g of colorless viscous liquid, yield: 95%.
[0052] 1 H NMR (400MHz, CDCl 3 ):δ3.53(t,J=6.9Hz,2H),2.89(s,3H),2.61(t,J=6.8Hz,2H),1.47(s,9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com