Gelatin composition, and preparation method and application thereof
A composition and gelatin technology, applied in the field of medicine, can solve problems such as poor hemostatic effect, and achieve the effects of strong functionality, low antigenicity and allergenicity, and enhanced elasticity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 genetic engineering method prepares microbial transglutaminase (MTG)
[0034] The wild MTG strain gene is shown in sequence 1 (SEQ ID NO.1), and the MTG strain gene used in the present invention is shown in sequence 2 (SEQ ID NO.2). G at position 73 is changed to T, and T at position 750 is changed to g. The wild MTG amino acid sequence is shown in sequence 3 (SEQ ID NO.3), the MTG amino acid sequence used in the present invention is shown in sequence 4 (SEQ ID NO.4), the 25th Gly mutation is Cys, and the 250th Ser mutation for Arg. Design and commission a biotechnology company (such as Beijing Quanshijin Biotechnology Co., Ltd., Shanghai Bioengineering Company) to synthesize the MTG strain gene of the sequence shown in SEQ ID NO.2, and then clone it into the EcoRV of the Escherichia coli expression vector pET-32 site, and then transfer the expression vector to Escherichia coli BL21 to form engineering bacteria. The genetically engineered bacteria were c...
Embodiment 2
[0035] The fine purification of embodiment 2 microbial transglutaminase MTG
[0036] The supernatant obtained in Example 1 was filtered with a 0.22 μm filter membrane, and the filtrate was collected. Through a strong cation exchange column, the ion exchange column is GE's prepacked HiPrep16 / 10SP XL column, the flow rate is 2ml / min, and the sample is equilibrated with 50mM pH6.0 phosphate buffer before passing through the ion exchange column. The elution is 0-1M sodium chloride linear gradient elution, the elution peak is MTG at 100mM sodium chloride, the peak is collected, and then passed through the gel filtration column HiLoad16 / 60Superdex, the flow rate is 1ml / min, and 50mM pH6.0 Phosphate buffer eluted and the peaks were collected.
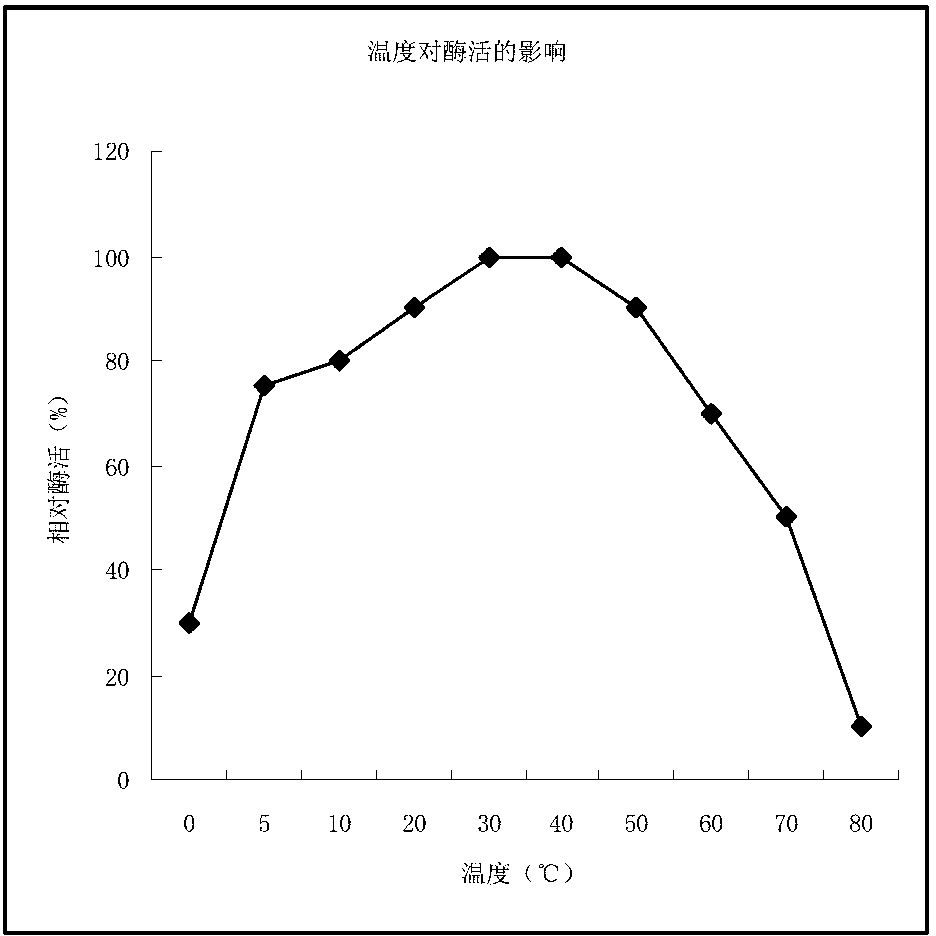
[0037] The purified MTG was tested for enzyme activity at different temperatures, and the experimental results were as follows: figure 1 as shown, figure 1 It shows that the optimal reaction temperature of the microbial transglutaminase of ...
Embodiment 3
[0039] The fermentation of embodiment 3 microbial transglutaminase MTG
[0040] Strain: Streptoverticillium mobaraense obtained by mutagenesis screening, the result obtained by sequencing is identical to the sequence of SEQ ID NO.1.
[0041] Mutagenesis method: Add 10ml of cold sterile seed medium to the eggplant bottle slant medium (Gaoshi No. 1 medium), scrape the surface mycelia fully with an inoculation needle, and pour it into a bottle containing 20-30 glass beads medium, 30°C, 200r / min shaking culture for 1h, so that the spores are in the state of germination. The operation is carried out under red light or dark conditions, and the ultraviolet light is turned on 0.5h in advance to stabilize the light source. Take 5 ml of the spore suspension diluted to 10 times and place it in a sterilized petri dish with a diameter of 9 cm and a magnetic stirrer for ultraviolet mutagenesis. The mutagenesis conditions are as follows: the power of the ultraviolet lamp is 15W, the irradi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com