A kind of acid-resistant high-temperature beta-amylase mutant and its application
An amylase and mutant technology, which is applied in the application field of producing high-purity maltose syrup, can solve the problem that β-amylase cannot take into account acid resistance and temperature resistance at the same time, and achieves the advantages of reducing production cost, reducing cost and simplifying process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] This example illustrates the acquisition of the β-amylase gene ctba and the construction of a recombinant expression vector
[0019] 1) Extract Thermoanaerobacterium thermosulfurigenes, American Type Culture Collection No. ATCC33743, chromosomal DNA as a template;
[0020] 2) Use SEQ ID NO: 2 as the upstream primer (containing a Nco I restriction site) and SEQ ID NO: 3 (containing a Bam HI restriction site and introducing a 6x histidine tag coding sequence) as The downstream primers are used to amplify the mature peptide coding region of the β-amylase gene ctba, whose GenBank sequence number is M22471.1, by polymerase chain reaction PCR, corresponding to the 660-2219 fragment in the ctba gene, and the length of the obtained PCR target product is 1605bp ;
[0021] 3) The target product and the pSE380 vector plasmid were double-digested with Nco I and Bam HI enzymes respectively, after the gel was recovered, ligated with T4DNA ligase, and transformed into E. coli (E.coli...
Embodiment 2
[0024] This example illustrates the construction of mutant expression plasmids
[0025] Using the PCR primer-mediated site-directed mutagenesis method, the whole plasmid pSBA DNA was used as a template to construct a single-point mutant, and then the recombinant plasmid containing the single-point mutation was used as a template to introduce the second mutant with primers containing the second mutation site. Mutation, and so on to construct mutant plasmids containing multiple mutation sites. details as follows:
[0026] 1) Use pSBA plasmid DNA as a template, and use SEQ ID NO:4 and SEQ ID NO:5 as upstream and downstream primers respectively to construct a PCR reaction system. The 25 μL PCR reaction system was constructed as follows: 1 μL pSA7D plasmid DNA, 0.5 μL upstream primer Amy7-S (concentration: 10 mmol / L), 0.5 μL downstream primer Amy7-A (concentration: 10 mmol / L), 2 μL dNTPs (per dNTP2.5mmol / L), 5μL5x buffer, 0.25μL (2.5U / μL) DNA polymerase, add 16.75 μL ddH 2O ...
Embodiment 3
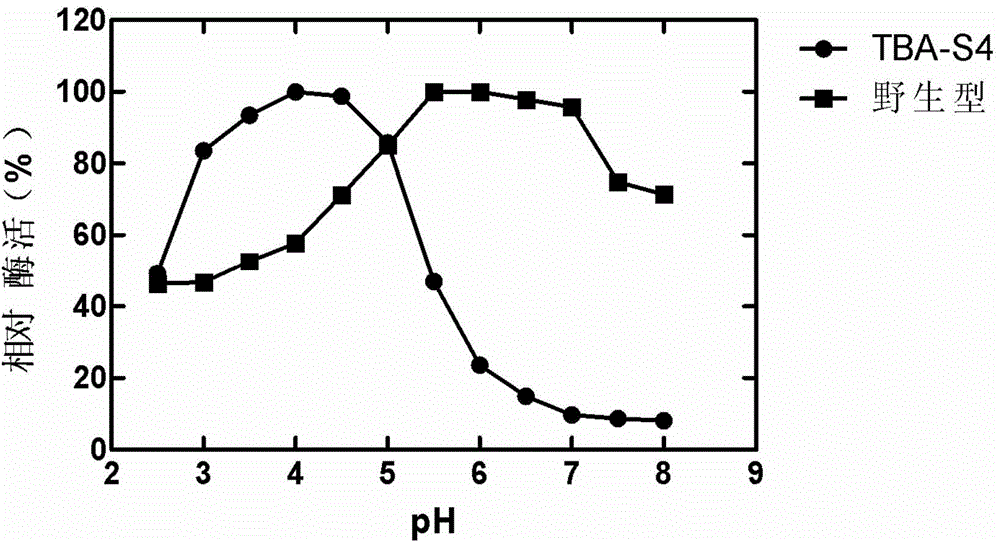
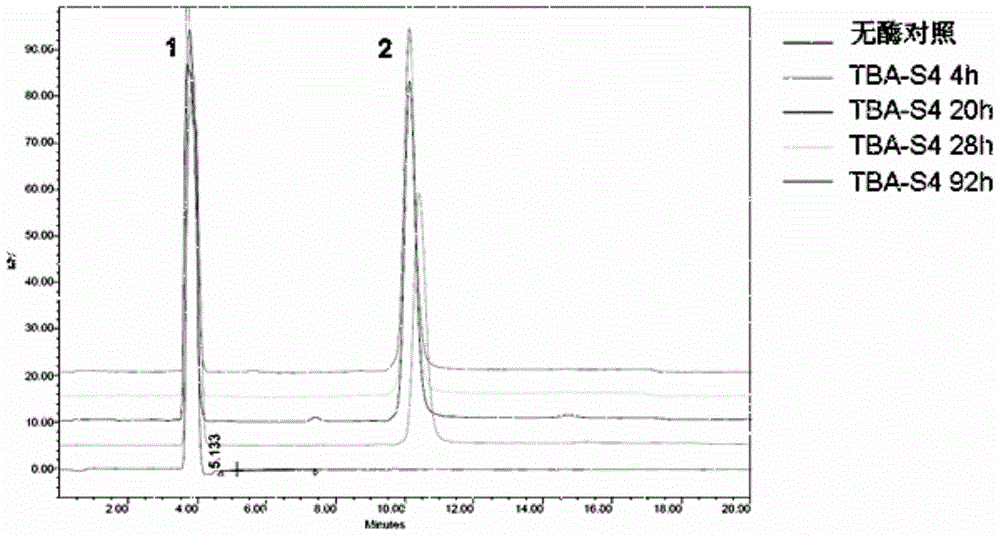
[0031] This example illustrates the induction, expression, purification and characterization of mutant enzymes
[0032] 1) Pick a single colony of Escherichia coli engineering bacteria containing the recombinant expression plasmid pSBA-S4, and inoculate it in 5ml LB culture medium containing 100μg / ml ampicillin (yeast extract 10g / L, peptone 5g / L, sodium chloride 10g / L , natural pH), cultured overnight at 37°C and 220r / min. The overnight cultured bacterial solution was transferred to 500ml LB culture solution containing 100μg / mL ampicillin according to the inoculum size of 1%. When the bacterial solution was cultured to OD 600 is about 0.6, add IPTG inducer with a final concentration of 1mmol / L, and continue to induce culture for 16h under the same conditions;
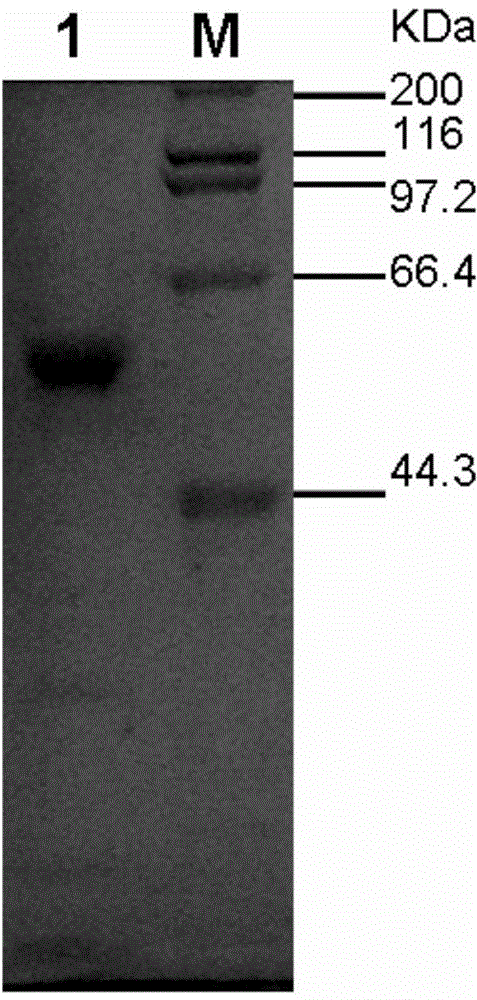
[0033] 2) Centrifuge at 9000r / min and 4°C to collect engineered bacteria that induce expression, wash the precipitate once with 0.05mol / L acetate buffer (pH6.0), resuspend in 100ml of the same buffer, and pass the bac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com