Multi-functional-group polyethylene oxide-b-aliphatic polyester block copolymers, preparing method thereof and applications thereof
A technology of aliphatic polyester and ethylene oxide, which is applied in pharmaceutical formulations, medical preparations of inactive ingredients, etc., can solve the problems of functional groups affecting polymerization, restricting promotion and application, and difficulty in monomer synthesis, and achieves the reaction conditions. Controllable, easy-to-operate, and easy-to-quantify effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1: the synthesis of poly(ethylene oxide-co-epichlorohydrin) random copolymer P(EO-co-ECH)
[0052] Add 3.0g of ethylene oxide and 0.7g of epichlorohydrin into the reaction flask at about 0°C, add 25mL of dichloromethane, and then add 0.75mL of 1.0M tetrahydrofuran solution of potassium triisobutyloxide, triisobutylaluminum Catalyst 1.5 mL. After 4 hours of reaction, the temperature was raised to room temperature and the reaction was continued for 24 hours. All the above operations ensure that the reaction system is anhydrous and oxygen-free. Add 200 μL of ethanol to stop the reaction. After most of the solvent was removed by rotary evaporation, it was precipitated in 200 mL of ice methanol to obtain 3.2 g of the product poly(ethylene oxide-co-epichlorohydrin) with a yield of 86%. Its molecular weight was measured by gel permeation chromatography (see Figure 7 ), the chemical structure was confirmed by proton NMR spectroscopy. The number average molecula...
Embodiment 2
[0053] Embodiment 2: Synthesis of P(EO-co-Glycidyl Azide)
[0054] Dissolve 2.0 g of poly(ethylene oxide-co-epichlorohydrin) random copolymer with a number average molecular weight of 5000 (where the molar ratio of ethylene oxide to epichlorohydrin is 9 / 1) in 15 mL of nitrogen , nitrogen-dimethylformamide (DMF), adding sodium azide (NaN 3 ) 2.0g, refluxed at 100°C for 48 hours under the protection of argon. After cooling to room temperature, the precipitate was removed by centrifugation, and the supernatant was precipitated in 200 mL of ice ether. The product was vacuum-dried to remove the organic solvent, dissolved in 10 mL of deionized water, transferred into a dialysis bag with a molecular weight cut-off of 3500 Da, and dialyzed for 48 hours, changing the water every 8 hours. After the dialysis was completed, the moisture was removed by freeze-drying to obtain 1.6 g of P(EO-co-Glycidyl Azide) product with a yield of 80%. Its molecular weight was measured by gel permeatio...
Embodiment 3
[0055] Embodiment 3: the synthesis of the amphiphilic block copolymer containing P (EO-co-Glycidyl Azide) block
[0056] (1) Synthesis of PCL-b-P (EO-co-Glycidyl Azide)
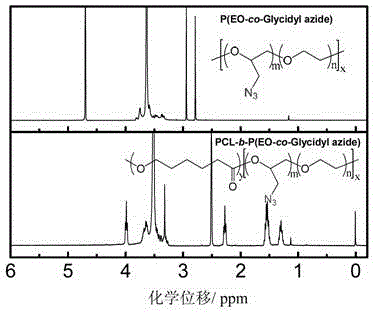
[0057] 2.0 g of P (EO-co-Glycidyl Azide) prepared in Example 2 (wherein the ratio of ethylene oxide to azidopropylene oxide is 9 / 1) was placed in the reaction flask, and the reaction system was repeatedly pumped out to remove the Sn(Oct) with a concentration of 0.982mol / L was sequentially added after oxygen 2 Toluene solution 5.0μL, caprolactone 2.0g, toluene 12mL. The closed reaction system was reacted at 110°C for 48 hours, cooled to room temperature, and precipitated in 300 mL of glacial ether to obtain 3.6 g of PCL-b-P (EO-co-Glycidyl Azide) block copolymer with a yield of 90%. Its molecular weight was measured by gel permeation chromatography (see Figure 9 ), the chemical structure was confirmed by proton NMR spectroscopy (see Figure 10 ).
[0058] After measurement, the number average molecular w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com