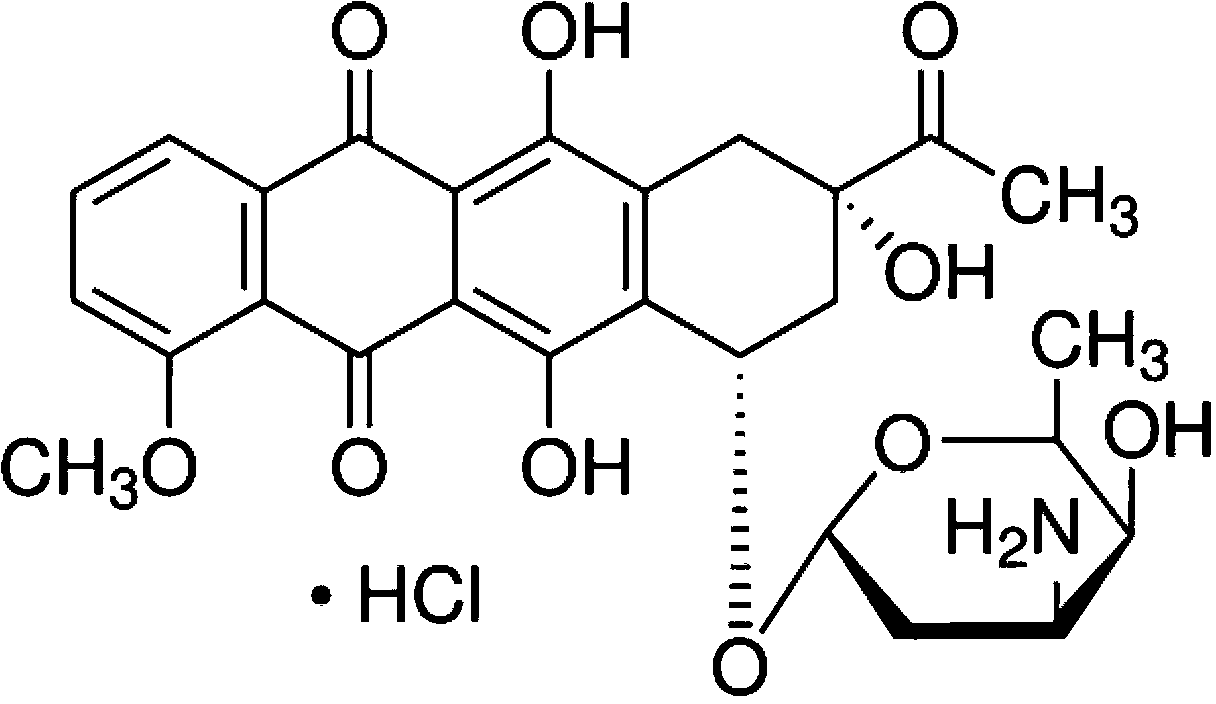
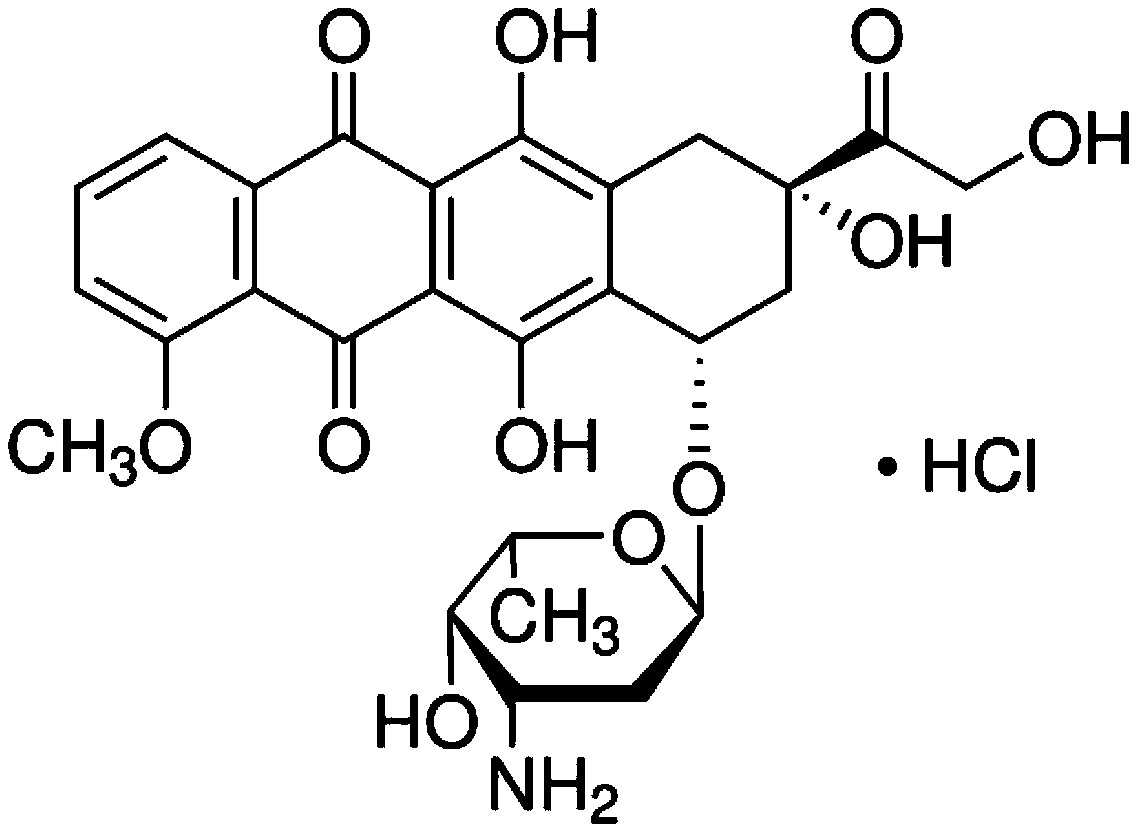
Daunorubicin C-14 hydroxylase mutant and production method of genetically engineered bacteria thereof
A hydroxylase mutant, a technology of genetically engineered bacteria, applied in genetic engineering, microorganism-based methods, biochemical equipment and methods, etc., can solve problems such as low productivity and pollution of the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Step Q1: Cloning of the C-14 hydroxylase gene of Streptomyces C5
[0079] Q1: The cloning method of the C-14 hydroxylase gene of Streptomyces C5, comprising the following sub-steps:
[0080] Q11: PCR amplification:
[0081] Q111: Design a pair of primers, where the underlined part is the introduced BamHI site and HindIII site:
[0082] 5'-CT GGATCC ATGGGCGGTGGGCGGTCC-3' and
[0083] 5'-AT AAGCTT CACGGGGCCGGCTTCTCG-3';
[0084] Q112: Using the Streptomyces C5 cell as a template, design PCR amplification primers for PCR amplification. The PCR system is: 2μl PCR buffer (that is, the buffer of the PCR reaction, the purpose is to provide an optimal enzymatic reaction condition); 25mMol / L MgCl 2 1μl, 2.5mMol / L dNTP1.5μl, take 1μl of the two primers in the above Q111 step, pick a small amount of Streptomyces C5 cells as a template with a toothpick, 2 units of Taq enzyme (purchased by Shanghai Sangon Company), two Methyl sulfoxide 1 μl, and finally make up to 20 μl wi...
Embodiment 2
[0092] Step Q2: Random mutations to the doxA gene
[0093] Q21: Take the primer pair:
[0094] 5'-AGCCTGAATCACTTCCGAATTCA-3' and
[0095] 5'-TTCATTGGACCTAATACCGATCA-3';
[0096] Q22: Take 50 μl of the error-prone PCR reaction system, including: 20 ng of the E. coli plasmid containing the doxA gene after the completion of the Q1 step, 30 pmol each of a pair of primers in the Q21 step, 7 mMol / LMgCl 2 , 50mMol / L KCl, 10mMol / L Tris-HCl, (pH 8.3), 0.2mMol / L dGTP, 0.2mMol / L dATP, 1mMol / L dCTP, 1mMol / L dTTP, 0.05mMol / L MnC1 2 , and 5 enzyme activity units of Taq enzyme (Shanghai Sangon Company);
[0097] Q23: Set the PCR reaction conditions as follows: denature at 94°C for 10 minutes, then maintain denaturation at 94°C for 30 seconds, then anneal at 72°C for 30 seconds, and extend at 72°C for 2 minutes;
[0098] Q24: Repeat step Q23 for 30 cycles, and then fully extend for 10 minutes at 72°C;
[0099] Q25: Use 1% agarose gel to perform nucleotide electrophoresis at a voltage of ...
Embodiment 3
[0106] Q3: Screening of mutant library:
[0107] Q31: Transformation of mutants: transform the mutants constructed after step 2 into competent cells E.coli DH10B by electric shock method (Invitrogen);
[0108] Q32: Spread the competent E.coli DH10B cells transformed in step Q31 on LB plates containing ampicillin antibiotics, and culture them at 37°C; the LB plates also contain 1% peptone, yeast extract 0.5% sodium chloride, 1% sodium chloride, 2% agar;
[0109] Q33: Screening and identification of mutants:
[0110] Q331: Pick the bacteria grown on the LB plate and transfer them to liquid LB test tubes, culture them overnight in a shaker at 220 rpm in a culture environment at 37°C, and transfer them to 50ml LB the next day In the 250ml shake flask of the culture medium, after 3 hours, the bacterium was induced with 1mMIPTG, after continuing to cultivate for 18h, after centrifugation at 4000 rpm for 15 minutes, the bacterium was collected;
[0111] Q332: Carry out daunorubici...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Enzyme activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com