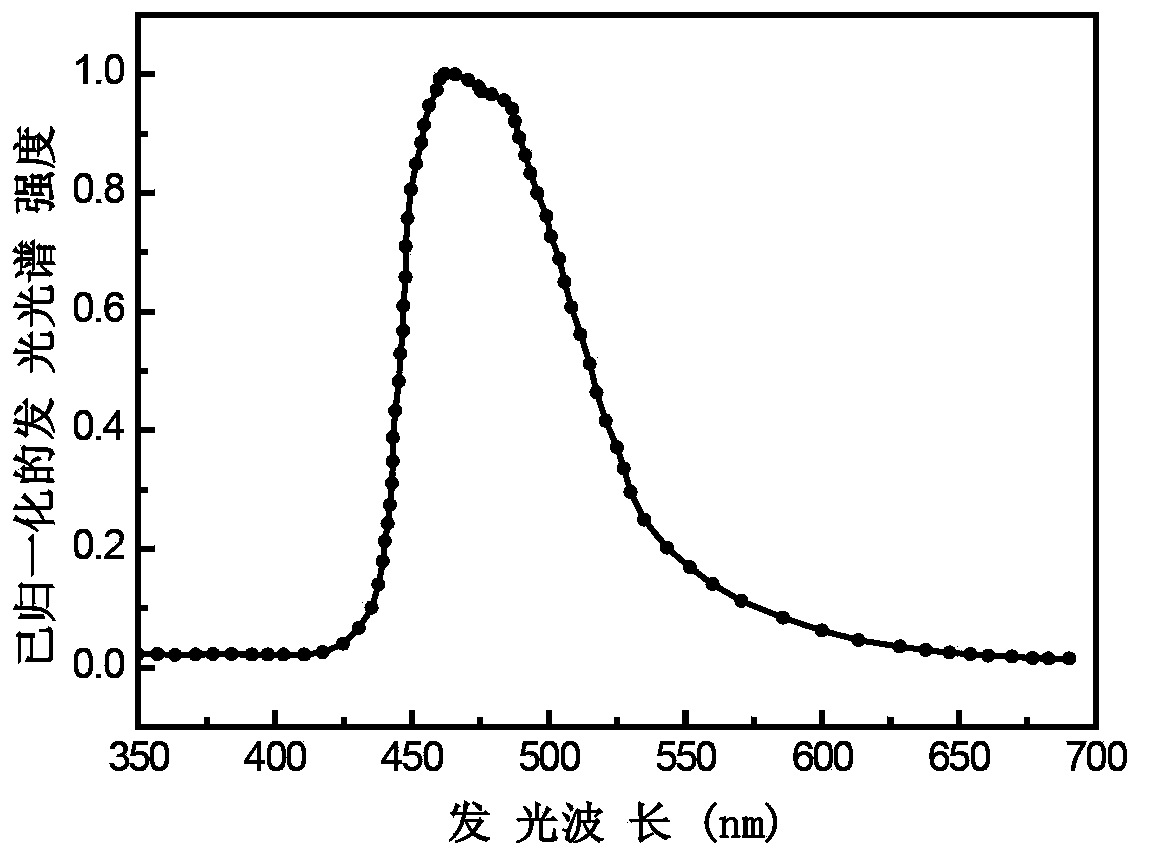
Blue phosphorescence iridium metal complex, preparation method and organic electroluminescent device
A technology of iridium metal complexes and blue phosphorescence, which is applied in the fields of electric solid-state devices, organic chemistry, and luminescent materials, can solve the problems of lagging development and poor luminous performance of light-emitting devices, and achieve increased luminous intensity, improved luminous performance, and improved The effect of luminous properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
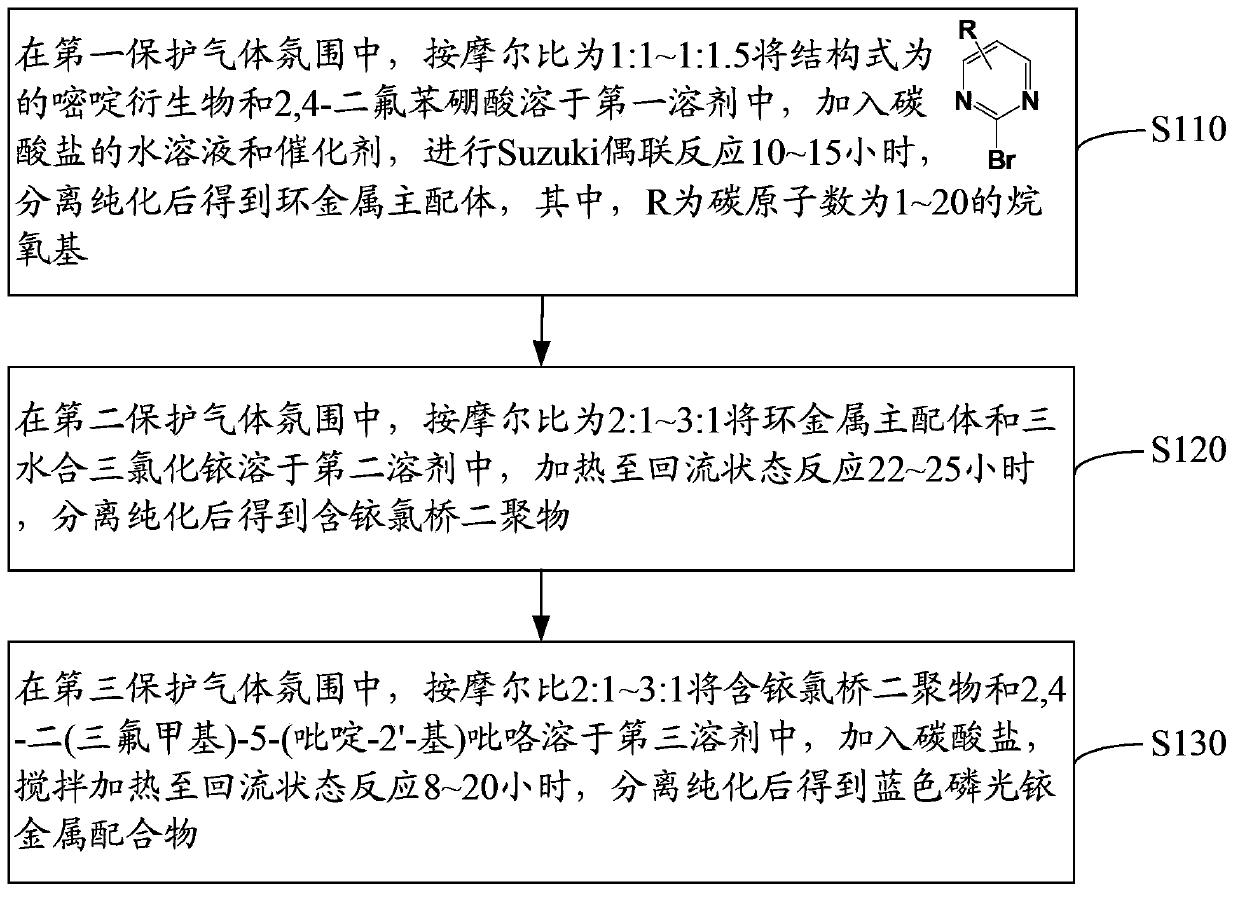
[0065] The preparation method of the blue phosphorescent iridium metal complex has mild reaction conditions, low equipment requirements, low preparation cost, and easy large-scale preparation.
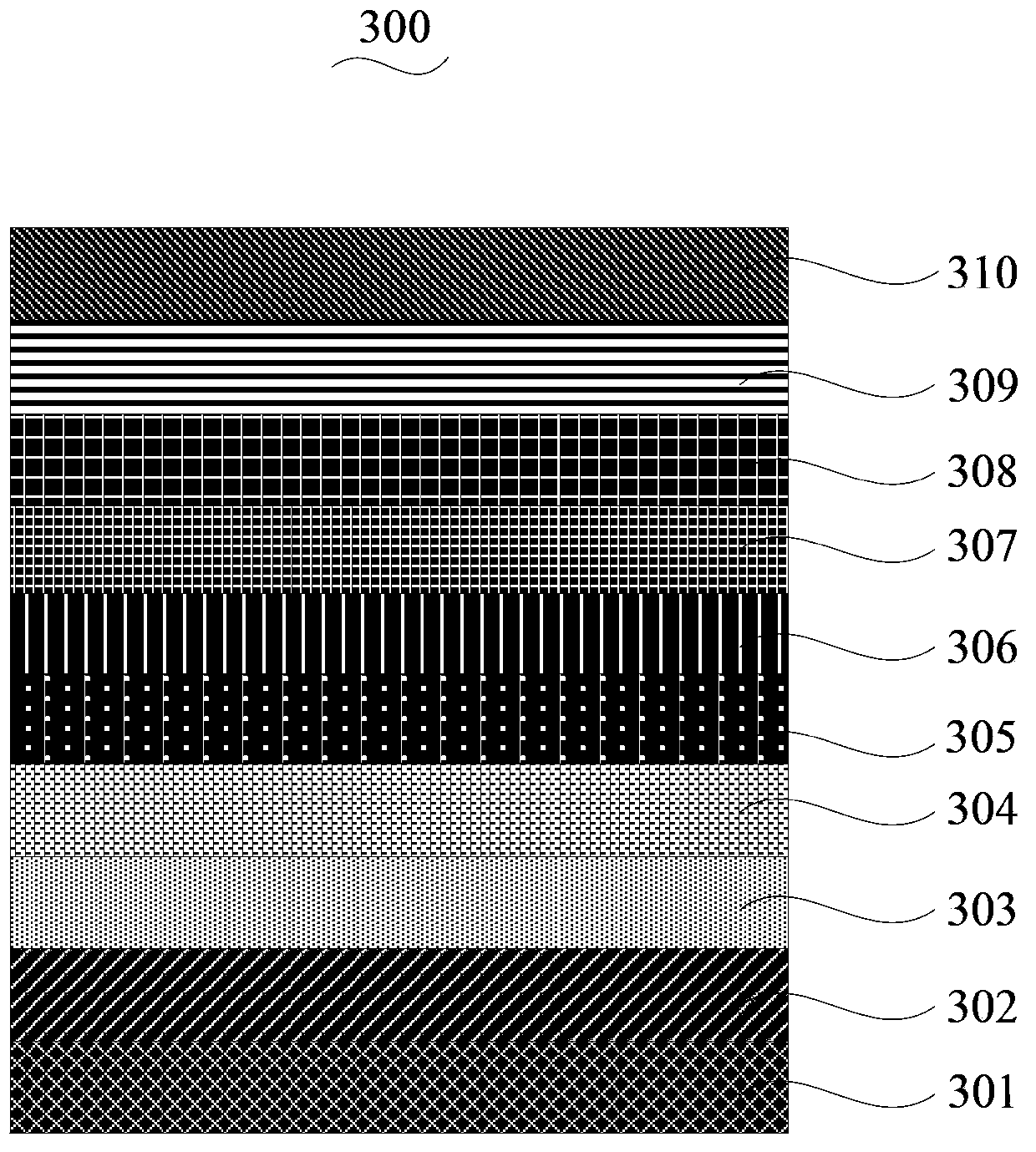
[0066] See figure 2 , The organic electroluminescent device 300 of an embodiment includes a substrate 301, an anode 302, a hole injection layer 303, a hole transport layer 304, an electron blocking layer 305, a light emitting layer 306, a hole blocking layer 307, and electrons which are sequentially stacked. The transport layer 308, the electron injection buffer layer 309 and the cathode 310.
[0067] The materials of the substrate 301, the anode 302, the hole injection layer 303, the hole transport layer 304, the electron blocking layer 305, the hole blocking layer 307, the electron transport layer 308, the electron injection buffer layer 309, and the cathode 310 are commonly used in the field. material. For example, the substrate 101 is a glass substrate, the material of the anode 302 i...
Embodiment 1
[0072] Blue phosphorescent bis(2-(4',6'-difluorophenyl)-5-methoxypyrimidine-N,C 2' ) Synthesis of (2,4-bis(trifluoromethyl)-5-(pyridine-2'-yl)pyrrole) iridium complex.
[0073] Blue phosphorescent bis(2-(4',6'-difluorophenyl)-5-methoxypyrimidine-N,C 2' The structural formula of (2,4-bis(trifluoromethyl)-5-(pyridine-2'-yl)pyrrole) iridium complex is as follows:
[0074]
[0075] (1) Synthesis of 2-(2',4'-difluorophenyl)-5-methoxypyrimidine
[0076] In a nitrogen atmosphere, 1.89g (10mmol) 2-bromo-5-methoxypyrimidine, 1.89g (12mmol) 2,4-difluorophenylboronic acid and 0.58g (0.5mmol) tetrakis (triphenylphosphorus) Palladium was dissolved in 35 mL of toluene, and then 15 mL of an aqueous solution containing 2.76 g (20 mmol) of potassium carbonate was added dropwise to the reaction system. Heating, stirring and reacting at 90°C for 10h. After the reaction is over, after the reaction solution is cooled to room temperature, extract with dichloromethane, take the organic phase, wash with w...
Embodiment 2
[0102] Blue phosphorescent bis(2-(4',6'-difluorophenyl)-5-hexoxypyrimidine-N,C 2' ) Synthesis of (2,4-bis(trifluoromethyl)-5-(pyridine-2'-yl)pyrrole) iridium complex.
[0103] Blue phosphorescent bis(2-(4',6'-difluorophenyl)-5-hexoxypyrimidine-N,C 2' The structural formula of (2,4-bis(trifluoromethyl)-5-(pyridine-2'-yl)pyrrole) iridium complex is as follows:
[0104]
[0105] (1) Synthesis of 2-(2',4'-difluorophenyl)-4-hexoxypyrimidine
[0106] In an argon atmosphere, 1.30g (5mmol) 2-bromo-4-hexoxypyrimidine, 0.79g (5mmol) 2,4-difluorophenylboronic acid and 0.14g (0.2mmol) dichlorobis(triphenyl) Phosphorus) palladium was dissolved in 30 mL of DMF, and then 15 mL of an aqueous solution containing 2.07 g (15 mmol) of potassium carbonate was added dropwise to the reaction system. Heat, stir and react under reflux at 80°C for 12 hours. The reaction is over. After the reaction solution is cooled to room temperature, extract with dichloromethane, take the organic phase, wash with water un...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com