Preparation method of tetramethylbiphenyl isomers
A technology of tetramethylbiphenyl and isomer, which is applied in the field of preparation of tetramethylbiisomer, can solve the problems of low catalyst efficiency, unfavorable industrial production, large industrial waste residue, etc., and achieves improved conversion rate and reaction yield. efficiency, low price, and reduced operating environment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
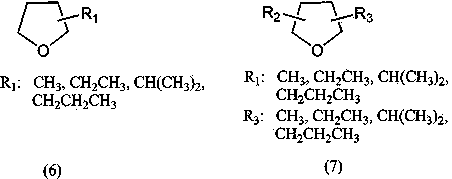
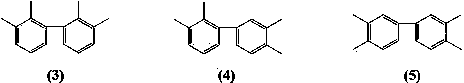
[0038] Mix 4-bromo-o-xylene (36.8 g, 0.2 moL), magnesium ribbon (1.2 g, 0.05 moL), anhydrous 2-methyl-substituted tetrahydrofuran (52 g, 0.6 moL), iodine (5 mg, 0.02 mmoL), Anhydrous NiCl 2 (0.064 g, 0.5 mmoL), mixed in a nitrogen atmosphere, 90 o C reacted for 8 hours, cooled, filtered off the magnesium bromide precipitate, and the filtrate was distilled to recover 49 g of solvent 2-methyltetrahydrofuran, recovered excess brominated o-xylene 18.5 g, and collected 145 to 155 g of brominated o-xylene by distillation under reduced pressure. o C fraction (pressure 1~4 mmHg). 19.2 g of 3, 3’, 4, 4’-tetramethylbiphenyl was obtained with a yield of 91% (based on magnesium) and a melting point of 74-75 o c.
Embodiment 2
[0040] 3-Chloro-o-xylene (28 g, 0.2 moL), magnesium ribbon (1.2 g, 0.05 moL), anhydrous 2-methyl-substituted tetrahydrofuran (43 g, 0.5 moL), isopropylmagnesium chloride (20.4 mg, 0.2 mmol), anhydrous NiCl 2 (0.064 g, 0.5 mmoL), bipyridine (0.078 g, 0.5 mmoL) were mixed in a nitrogen atmosphere, heated to reflux (~115 o C) After reacting for 8 hours, cool down, filter out the magnesium chloride precipitate, and recover 40 g of 2-methyltetrahydrofuran solvent from the filtrate by distillation, recover 13.5 g of excess 3-chloro-o-xylene, collect 130~145 o Fraction C (pressure 1~3 mmHg), obtained 17.9 g of 2, 2’, 3, 3’-tetramethylbiphenyl, with a yield of 85% (based on magnesium), and a melting point of 115~116 o c.
Embodiment 3
[0042] 4-Chloro-o-xylene (14 g, 0.1 moL), magnesium ribbon (2.4 g, 0.1 moL), anhydrous 2,5-dimethyltetrahydrofuran (50 g, 0.5 moL), iodine (2.5 mg, 0.01 mmoL), reflux under argon atmosphere (~110 o C) React until the metal magnesium is completely dissolved, that is, the Grignard reagent is completely generated, and then add the catalyst anhydrous NiCl 2 (0.13 g, 1 mmol) and triphenylphosphine (0.26 g, 1 mmol), then added 3-chloro-o-xylene (14 g, 0.1 moL), ~110 o C carried out the coupling reaction for 7 hours, then cooled and filtered off the inorganic salt precipitate, and the filtrate was distilled to recover 46 g of 2,5-dimethyltetrahydrofuran solvent, and 140 to 145 g was collected by vacuum distillation. o Fraction C (pressure 1~3 mmHg), 18.5 g of 2,3',3,4'-tetramethylbiphenyl was obtained with a yield of 88% (based on magnesium), and a melting point of 45~46 o c.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com